(a) Give descriptions of the bonding in ClO 2 and [ClO 2 ] (17.24 and 17.35),...
Question:
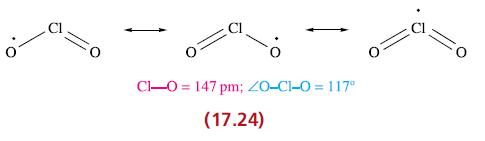
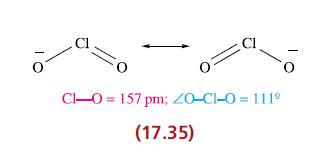
(a) Give descriptions of the bonding in ClO2 and [ClO2]− (17.24 and 17.35), and rationalize the differences in Cl—O bond lengths.
(b) Rationalize why KClO4 and BaSO4 are isomorphous.
Transcribed Image Text:
O Cl-0= 147 pm; 20-C1-0 = 117° (17.24)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
a Bonding in ClO2 and ClO2 ClO2 Chlorine dioxide Chlorine dioxide is a paramagnetic bent molecule with a structure like AX2E The central chlorine atom ...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The oxyanion of nitrogen in which it has the highest oxi-dation state is the nitrate ion (NO3-). The corresponding oxyanion of phosphorus is PO43-. The NO43- ion is known but is not very stable. The...
-
The NMR spectra below are for the organic compounds C6H12 and C4H10O. Deduce the structures for these compounds. See Exercise 70 for a discussion of the bonding in organic compounds. The structure of...
-
The melting and boiling points of the titanium tetrahalides are given below. Rationalize these data in terms of the bonding in and the intermolecular forces among these compounds. bp (C) mp (C) 284...
-
This is one question with sub parts please solve this question step by step please also write the little explanation to solve the question Consider the following complex numbers: z, = 3+ 3i z2 = 3 +...
-
In the Home Depot 2009 financial statements in Appendix A at the end of this textbook, read note 1. Find the information about Home Depots international store locations. a. In what countries (other...
-
Problems show the derivative f' of f. (a) Where is f increasing and where is f decreasing? What are the x-coordinates of the local maxima and minima of f? (b) Sketch a possible graph for f. (You dont...
-
1. Discuss the importance of Financial Management.
-
Each of the four independent situations below describes a direct financing lease in which annual lease payments of $10,000 are payable at the beginning of each year. Each is a capital lease for the...
-
Application of Time Value of Money Skills Nick Nohitter has been playing baseball since he was five years old and has always dreamed of playing in the big leagues. Last season, he was a starting...
-
Figure 17.15 shows a FrostEbsworth diagram for chlorine. (a) How is this diagram related to the potential diagram for chlorine in Fig. 17.14? (b) Which is the most thermodynamically favoured species...
-
The electronic spectra of mixtures of CH 2 Cl 2 solutions (each 0.993 mmol dm 3 ) of I 2 and the donor D shown in the diagram after the table were recorded for different volume ratios of the two...
-
Describe in words the motion of the graphed in Fig. 2-29. 40 30 20 10 80 90 100 110 120 20 30 40 50 60 70 10 20 30 I (s) (s/u) a
-
The combined weight of the load and the platform is 200 lb, with the center of gravity located at G. If a couple moment of M = 900 lb ft is applied to link AB, determine the angular velocity of links...
-
Due In: 06:48:23 Questions Question 1 (4) O Question 2 (8) Question 2 of 2 A company sold $150,000 bonds and set up a sinking fund that was earning 8.5% compounded semi-annually to retire the bonds...
-
Find the point on the graph of f(x) = x which is closest to the point (6, 27). How close is the closest point?
-
Due to a crash at a railroad crossing, an overpass is to be constructed on an existing level highway. the existing highway has a design speed of 50 mi/h. The overpass structure is to be level,...
-
Finding Bone Density Scores. In Exercises 37-40 assume that a randomly selected subject is given a bone density test. Bone density test scores are normally distributed with a mean of 0 and a standard...
-
Prepare a 1,400 word paper in which you compare and contrast Abraham Maslow (Humanistic) theories to Carl Jung (psychodynamic) theories. Address the following items: Describe the role of personality...
-
What is beacon marketing? What are digital wallets?
-
Which metal carbonyl in each of (a) [Fe(CO) 4 ] 2 or [Co(CO) 4 ] , (b) [Mn(CO) 5 ] or [Re(CO) 5 ] should be the most basic towards a proton? Explain your answer.
-
What conclusions can you draw from the bonding and reactivity of dihydrogen bound to a low-oxidation-state d-block metal that might be applicable to the bonding of an alkane to a metal? What...
-
Using the 18-electron rule as a guide, indicate the probable number of carbonyl ligands in (a) [W( 6 -C 6 H 6 )(CO) n ], (b) [Rh( 5 -C 5 H 5 )(CO) n ], (c) [Ru 3 (CO) n ].
-
Arnold inc. is considering a proposal to manufacture high end protein bars used as food supplements by body builders. The project requires an upfront investment into equipment of $1.4 million. This...
-
Billy Bob bank has three assets. It has $83 million invested in consumer loans with a 3-year duration, $46 million invested in T-Bonds with a 12-year duration, and $69 million in 6-month (0.5 years)...
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...

Study smarter with the SolutionInn App


