(a) The reaction of cis-[PtMe 2 (Me 2 SO)(PPh 3 )] with pyridine leads to cis-[PtMe 2...
Question:
(a) The reaction of cis-[PtMe2(Me2SO)(PPh3)] with pyridine leads to cis-[PtMe2(py)(PPh3)] and the rate of reaction shows no dependence on the concentration of pyridine. At 298 K, the value of ΔS‡ is 24 JK−1 mol−1. Comment on these data.
(b) For the reaction:
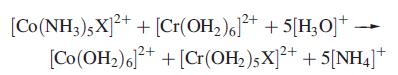
rate constants for X = Cl− and I− are 6:0 × 105 and 3:0 × 106 dm3 mol−1 s−1, respectively. Suggest how the reactions proceed and state which step in the reaction is the rate-determining one. Comment on why the rate constants for X− = Cl− and I− differ.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





