(a) The reaction of CsF, I 2 O 5 and IF 5 at 435K leads to Cs...
Question:
(a) The reaction of CsF, I2O5 and IF5 at 435K leads to Cs2IOF5. When the amount of CsF is halved, the product is CsIOF4. Write balanced equations for the reactions. Are they redox reactions?
(b) Using data in Fig. 17.14, calculate ΔG°(298 K) for the reaction:
![]()
Comment on the fact that the reaction does not occur at 298 K.
(c) Chlorine dioxide is the major bleaching agent in the pulp industry. While some statistics for bleaching agents list ClO2, others give NaClO3 instead. Suggest reasons for this difference.
Figure 17.14.
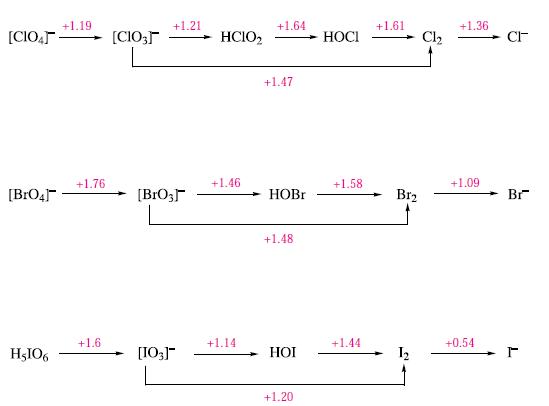
Transcribed Image Text:
4[C103] (aq) = 3[C104]¯¯(aq) + Cl¯ (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
a Balanced equations for the reactions Reaction at 435K2 CsF I2O5 5 IF5 Cs2IOF5 When the amount of CsF is halvedCsF I2O5 4 IF5 CsIOF4 2 F2 Regarding w...View the full answer

Answered By

Diana Muriuki
As an online math tutor, I have several years of hands-on experience working with students of all ages and skill levels. I hold a Bachelor's degree in Mathematics and a Master's degree in Education. Additionally, I have completed multiple training courses in online teaching and tutoring methods.
Throughout my career, I have worked with students in both individual and group settings, including classroom teaching, after-school tutoring, and online instruction. I am proficient in teaching a wide range of math topics, from basic arithmetic to advanced calculus and statistics.
One of my greatest strengths as a tutor is my ability to adapt my teaching style to meet the unique needs and learning styles of each individual student. I understand that every student is different, and I strive to create a comfortable and supportive learning environment that encourages growth and development.
In addition to my formal education and tutoring experience, I am also a lifelong learner with a passion for mathematics. I am constantly seeking out new resources and methods to improve my own knowledge and skills, and I believe this passion and enthusiasm helps to inspire my students as well.
Overall, my hands-on experience and proficiency as a math tutor are grounded in a combination of formal education, practical experience, and a genuine love of mathematics. I am confident in my ability to help students achieve their goals and succeed in math, and I look forward to the opportunity to work with new students and continue to grow as an educator.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The Bell Curve (Free Press, 1994), written by Richard Herrnstein and Charles Murray (H&M), is a controversial book about race, genes, IQ, and economic mobility. The book heavily employs statistics...
-
Write balanced equations for the three known reactions that transfer an amino group to a substrate by condensation with aspartate to give an inter-mediate that then undergoes an , -elimination to...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Suppose that In Example 18.6 the electrical firm does not have enough prior information regarding the population mean length of life to be able to assume a normal distribution for p. The firm...
-
Honda Motor Company reports that it has manufacturing facilities in over twenty locations around the world. Only four of those locations are in Japan. Several are in the U.S., Europe, and South...
-
Tell how many units and in what directions the graphs of the given equations are to be shifted. Give an equation for the shifted graph. Then sketch the original and shifted graphs together, labeling...
-
8. Distinguish between Profi t Maximisation and Wealth Maximisation objectives of the fi rm. [C.U. B.Com. (H), 2008]
-
Prior to the start of fiscal 2013, managers of MultiTech hosted a web conference for its shareholders, financial analysts, and members of the financial press. During the conference, the CEO and CFO...
-
Ons 6.8.90 Mayfair Company completed the following transactions and uses a perpetuntevernory wytem June 4 Sold 52.600 ot merchandise on credit that he cout 51.500 to W Wall June 5 Gold $29.000 of...
-
Suggest products for the following (which are not balanced): (a) [CIO3] + Fe+ + H+ (b) [103] + [SO3)- (c) [103] + Br + H+
-
Describe in outline how you would attempt: (a) To determine the equilibrium constant and standard enthalpy change for the aqueous solution reaction: (b) To show that the oxide I 4 O 9 (reported to be...
-
Monitor employee perceptions of the level of empowerment experienced? lop5
-
Primare Corporation has provided the following data concerning last month's manufacturing operations. Purchases of raw materials Indirect materials used in production Direct labor Manufacturing...
-
1. Start with the temperature at 1000 K. In the data table below, record the peak wavelength. Then, increase the temperature by 1000 K up to 10000 K and record the peak wavelength. Temperature (K)...
-
PROUT COMPANY AND SUBSIDIARYConsolidated Statements WorkpaperFor the Year Ended December 31, 2025Prout SextonEliminationsNoncontrollingConsolidatedCompanyCompanyDebitCreditInterestBalancesINCOME...
-
It is know that 4000 automobile trips are generated in a large residential area from noon to 1:00 P.M. on Saturdays fro shopping purposes. Four major shopping centers have the following...
-
dy 1. Find and simplify. dx tanx (a) y= (3 marks) (b) y x cosh (In x) (3 marks) (c) + sinh 2y = y - cosh 2x (4 marks)
-
One of the most important issues discussed in the collective bargaining process is seniority. It is one of those components of the workplace that seems to impact every worker, whether they are in the...
-
When is the indirect pattern appropriate, and what are the benefits of using it?
-
The rates of H 2 gas absorption (in dm 3 mol 1 s 1 ) by alkenes catalysed by [RhCl(PPh 3 ) 3 ] in benzene at 25C are: hexene, 2910; cis-4-methyl-2-pentene, 990; cyclohexene, 3160;...
-
Infrared spectroscopic investigation of a mixture of CO, H 2 , and 1-butene under conditions that bring about hydroformylation indicate the presence of compound (E) in Fig. 22.20 in the reaction...
-
(a) Starting with the alkene complex shown in Fig. 22.22 with trans-DHC=CHD in place of C 2 H 4 , assume dissolved OH attacks from the side opposite the metal. Give a stereochemical drawing of the...
-
Series of Compound Interest Techniques The following are several situations involving compound interest. Required: Using the appropriate table, solve each of the following: ( Click here to access the...
-
If Clark Kelly has recognized gain on an exchange of like-kind property held for investment use, where does Clark report the gain? First on Form 8824, then carried to Schedule D. First on Form 8824,...
-
An investor put 40% of her money in Stock A and 60% in Stock B. Stock A has a beta of 1.2 and Stock B has a beta of 1.6. If the risk-free rate is 5% and the expected return on the market is 12%,...

Study smarter with the SolutionInn App


