(a) Use Wades rules to rationalize the fact that in [Pd@Bi 10 ] 4+ , the Bi...
Question:
(a) Use Wade’s rules to rationalize the fact that in [Pd@Bi10]4+, the Bi atoms are arranged in a pentagonal antiprism. How is this structure related to that of [Pd@Pb12]2−?
(b) At 298 K, ammonium perchlorate decomposes according to the equation:
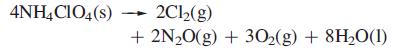
Determine ΔrG°(298 K) for this decomposition if ΔfG°(298 K) of N2O(g), H2O(l) and NH4ClO4(s) are +104, –237 and –89 kJ mol–1. What part does entropy play in the reaction?
Transcribed Image Text:
4NH4CIO4(s) 2Cl₂(g) + 2N₂O(g) + 30₂(g) + 8H₂O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (6 reviews)
ANSWER a Wades rules are a set of guidelines used to predict the structure of polyhedral boranes and metal cluster compounds based on the number of sk...View the full answer

Answered By

Collins Njuguna
I graduated from Maseno University with a Bachelor of Science in Applied Statistics. After graduation, I started tutoring students in mathematics. My experience in mathematics education is extensive and varied. I have taught a wide range of topics, including algebra, geometry, trigonometry, calculus, statistics, probability, and computer science. I have also worked with students of all ages and backgrounds, from elementary school to college.
My teaching method is based on the idea of hands-on learning. I believe that students learn best when they are actively engaged in the learning process, so I focus on giving students the tools they need to explore the material on their own. I also emphasize the importance of practice and review, as these are essential for mastering math concepts.
I have also developed several online and in-person courses on mathematics. My courses are designed to help students learn mathematics in an efficient and comprehensive way, and I use a variety of activities and exercises to ensure that my students are engaged and motivated.
Overall, my passion for mathematics and teaching has allowed me to be a successful tutor and educator in the field. I am confident that my experience will help your students master the mathematics they need to succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A simplified version of a commercial nuclear reactor involves fissile material such as enriched uranium 12 and a moderator such as graphite, both of which will be assumed in this exercise. Slow...
-
According to Van Luling what are some examples of how Trump's own rhetoric invokes the image and iconography of the Kong myth? In October of 2016, Daily Show host Trevor Noah compared Trump to Kong....
-
Please write an summary according to the article. Please don't copy from others and write full view of article in the summary. Value creation. Wealth creation. These are really powerful words. Maybe...
-
1. Read the following article about the new circle with disney product and answer the following questions. in doing so, take on the role as the marketing manager responsible for the product and...
-
John Marshall is employed as a bank loan officer for First State Bank. He is comparing two companies that have applied for loans, and he wants your help in evaluating those companies. The two...
-
Explain if Cloud based services has the potential and responsibility to fully replace other networks performance management technology in the future
-
When a parent buys or sells shares in its own subsidiary, how should the parent report the effects of such transactions in its consolidated financial statements? LO6
-
Solve for the Stackelberg subgame-perfect Nash equilibrium for the following game tree. What is the joint-profit maximizing outcome? Why is that not the outcome of this game? Leader Sets Output...
-
NOD Inc., a lessor, leased a drone to Worldz Information Network, Ltd., [WIN), a lessee, on January 1, 2019. The following information relates to the leased asset and the lease agreement: Fair value...
-
Predict the structures of (a) [NF 4 ] + ; (b) [N 2 F 3 ] + ; (c) NH 2 OH; (d) SPCl 3 ; (e) PCl 3 F 2 .
-
Deduce what you can about the nature of the following reactions. (a) One mole of NH 2 OH reacts with two moles of Ti(III) in the presence of excess alkali, and the Ti(III) is converted to Ti(IV). (b)...
-
For the following exercises, graph the functions. f(x) = x 2 2
-
Pop Company holds 70% of Son Company stock. Pop has sold inventory to Son Company as follows: Percent of Sold Sales Inventory Cost to Price to Held at Year Pop Son Year end 2018 $203,000 $355,000 30%...
-
A B C D E F G H J K L 1 Cost Mortgage Payments 2 Cost Description The upscale hotel's building was acquired for $10 million, leading to monthly mortgage payments of $60,000. Behavior Dollar Amount...
-
What celebrity attributes make for effective celebrity product endorsements? Celebrity testimonials are advertising messages delivered by famous people who say or imply that they use the...
-
Initial investment of $100,000 in a new medical equipment. Interest Rate 10% (Borrowed money from a bank). Item Year 0 Year 1 Year 2 Year 3 Year 4 Year 5 Investment 100,000 Expected Cash Flow 20,000...
-
How do I draw a top view of this sketch and I was also wondering how to draw an oblique cabinet projection. + + 1 1 ' '
-
When the local customs and laws conflict with the customs and laws of an organization operating abroad, which should prevail? Explain why.
-
The following cost information was provided to you for analysis: September 12,000 Units Produced Costs: TIC TAC TOE TING August 10,000 P80,000 70.000 60.000 50,000 How much is the fixed cost per...
-
In November 2006 the former KGB agent Alexander Litvinenko was found to have been poisoned by radioactive polonium-210. Write a review of the chemical and radiological properties of Po and discuss...
-
In their paper Formation of tellurium nanotubes through concentration depletion at the surfaces of seeds (Adv. Mater., 2002, 14, 279), B. Mayers and Y. Xia describe the synthesis of tellurium...
-
Correct any inaccuracies in the following statements and, after correction: (a) Elements in the middle of Group 16 are easier to oxidize to the group oxidation number than are the lightest and...
-
Assignment Title: The Role of Bookkeeping in Business Management and Financial Reporting Objective: Understand the importance of proper bookkeeping procedures in the management of...
-
17) The adjustment that is made to allocate the cost of a building over its expected life is called:A) depreciation expense.B) residual value.C) accumulated depreciation.D) None of the above answers...
-
9) Prepaid Rent is considered to be a(n):A) liability.B) asset.C) contra-asset.D) expense.10) As Prepaid Rent is used, it becomes a(n):A) liability.B) expense. C) contra-asset.D) contra-revenue.11)...

Study smarter with the SolutionInn App


