Arsenic is monotopic. What does this statement mean? Using Appendix 5, write down three other elements that
Question:
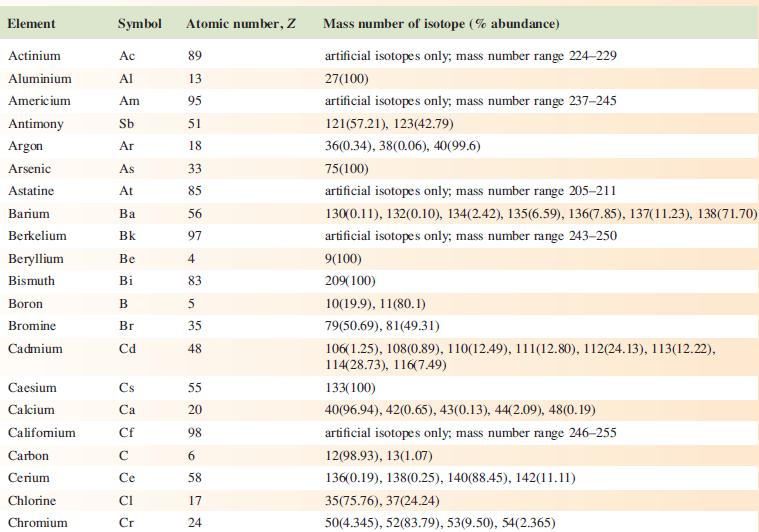
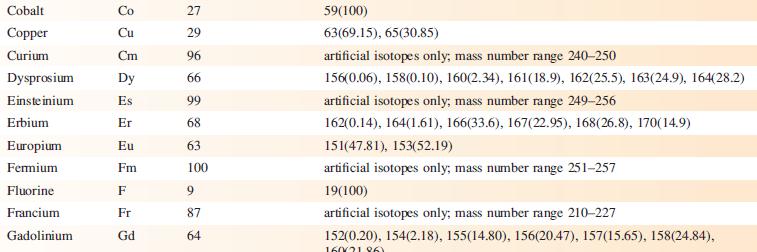
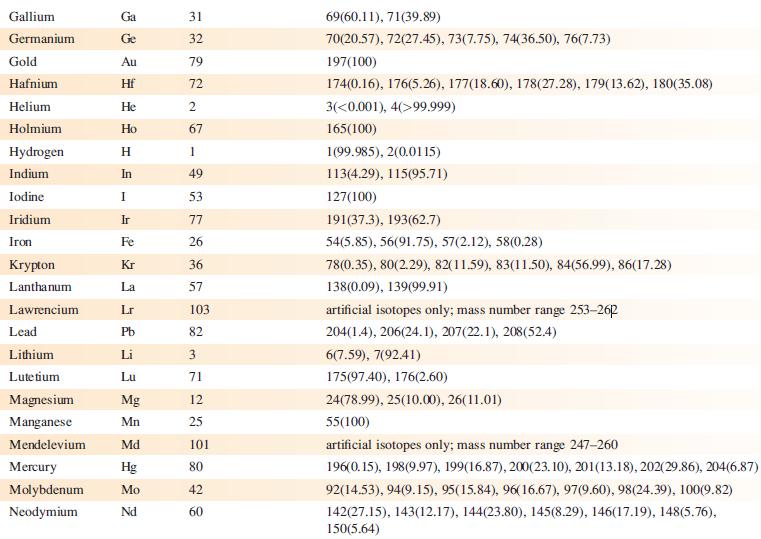
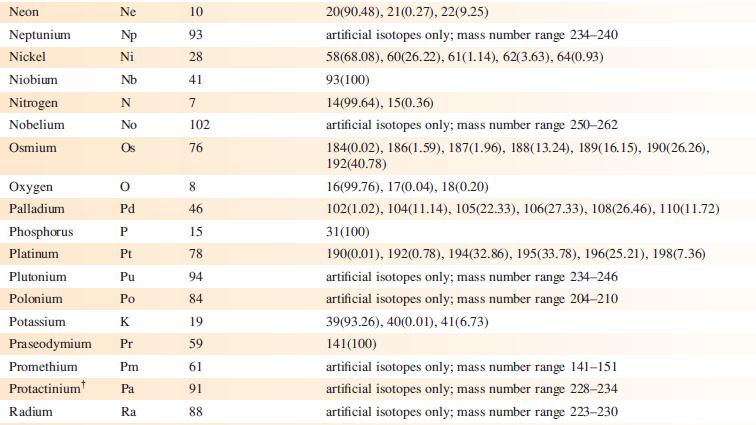
‘Arsenic is monotopic.’ What does this statement mean? Using Appendix 5, write down three other elements that are monotopic.
Data from Appendix 5
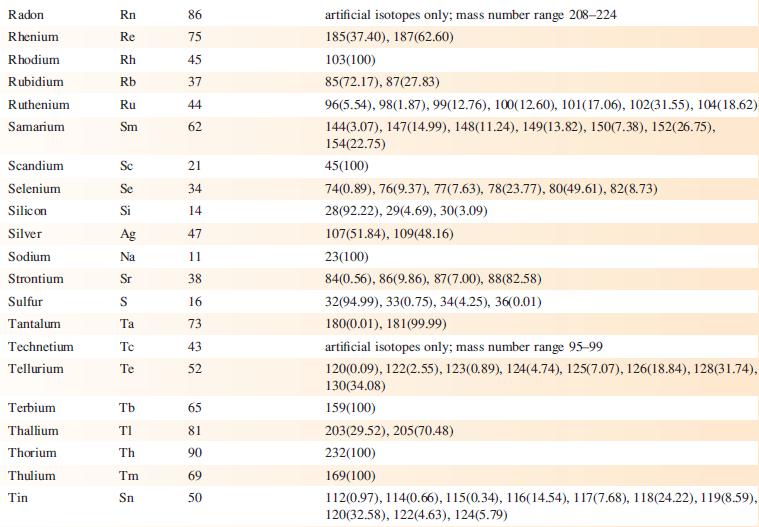
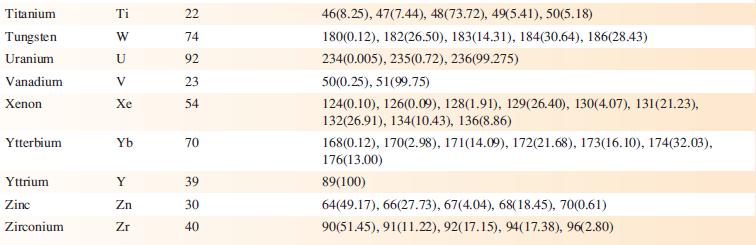
Transcribed Image Text:
Element Actinium Aluminium Americium Antimony Argon Arsenic Astatine Barium Berkelium Beryllium Bismuth Boron Bromine Cadmium Caesium Calcium Califomium Carbon Cerium Chlorine Chromium Symbol Atomic number, Z Ac Al Am Sb Ar As At Ba Bk Be Bi B Br Cd Cs Ca Cf с Ce CI Cr 89 13 95 51 18 33 85 56 97 4 83 5 35 48 55 20 98 6 58 17 24 Mass number of isotope (% abundance) artificial isotopes only; mass number range 224-229 27(100) artificial isotopes only; mass number range 237-245 121(57.21), 123(42.79) 36(0.34), 38(0.06), 40(99.6) 75(100) artificial isotopes only; mass number range 205-211 130(0.11), 132(0.10), 134(2.42), 135(6.59), 136(7.85), 137(11.23), 138(71.70)) artificial isotopes only; mass number range 243-250 9(100) 209(100) 10(19.9), 11(80.1) 79(50.69), 81(49.31) 106(1.25), 108(0.89), 110(12.49), 111(12.80), 112(24.13), 113(12.22), 114(28.73), 116(7.49) 133(100) 40(96.94), 42(0.65), 43(0.13), 44(2.09), 48(0.19) artificial isotopes only; mass number range 246-255 12(98.93), 13(1.07) 136(0.19), 138(0.25), 140(88.45), 142(11.11) 35(75.76), 37(24.24) 50(4.345), 52(83.79), 53(9.50), 54(2.365)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
Monotopic elements are those elements which possess only one naturally occurring nuclide or ...View the full answer

Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Youve just been hired onto ABC Company as the corporate controller. ABC Company is a manufacturing firm that specializes in making cedar roofing and siding shingles. The company currently has annual...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Case Study: Quick Fix Dental Practice Technology requirements Application must be built using Visual Studio 2019 or Visual Studio 2017, professional or enterprise. The community edition is not...
-
At the end of Year 1, Clayton Company had $7,000 of cash, $8,500 land, $2,500 of liabilities, $4,000 of common stock, and $9,000 of retained earnings. During Year 2, Clayton experienced the following...
-
Mint, Inc., restores antique automobiles. The retained earnings balance of the corporation was $27,000 at December 31, 2011. During 2012, the corporation paid $10,000 in dividends to its...
-
1. Find the exact value of the following logarithmic expression. (a) log, 2+ log, 32+ log, 64 (b) log, 81-3
-
During 2016 the tax agency fund collected and remitted $150,000 of the 2016 levies to the various governmental units. The collection fees associated with the $150,000 were remitted to Laramees...
-
At December 31,2016, Fako Travel Agency has an Accounts Receivable balance of $96,000. Allowance for Doubtful Accounts has a credit balance of $830 before the year-end adjustment. Service revenue...
-
12. (4 points) Pacific Manufacturing provided the following information for last month: Assume Pacific operates in the relevant range. If Pacific's Sales double next month what is the projected...
-
Hydrogen possesses three isotopes, but tritium ( 3 H), which is radioactive, occurs as less than 1 in 10 17 atoms in a sample of natural hydrogen. If the value of A r for hydrogen is 1.008, estimate...
-
Using the list of naturally occurring isotopes in Appendix 5, determine the number of electrons, protons and neutrons present in an atom of each isotope of (a) Al, (b) Br (c) Fe, and give appropriate...
-
Cytological examination of the sex chromosomes in a man has revealed that he carries an insertional translocation. A small segment has been deleted from the Y chromosome and inserted into the short...
-
As a job candidate in what way can I apply digital communications to ensure I thrive in the environment of recruiting by using social media for background checks
-
You hold a bond portfolio that consists of (i) a 4-year bond with a face value of $100 that pays an annual coupon of 10%, and (ii) a 2-year bond with a face value of $100 that pays an annual coupon...
-
Draw an original market equilibrium that describes the state of the market before the given scenario occurs. Clearly label both axis, label each a single supply curve and a single demand curve, and...
-
Analyze tools and/or metrics that a leader or manager should use to ensure that they are aligned and working together. Evaluate leadership strategies that could be employed to foster a positive...
-
Mexico has two main government programs that transfer income to rural households. PROCAMPO , which pays a set amount per acre to farmers who grew basic grains in a base year prior to the elimination...
-
Zillow.com is a site that can be used to assess the value of homes in your neighborhood. The organization provides a list of homes for sale as well as a Zestimate, which is the price Zillow believes...
-
The vapor pressure of the liquid NH, is measured at different temperatures. The following vapor pressure data are obtained. Temperature, K P, mmHg 217.1 223.4 234.7 588.1 Calculate the enthalpy of...
-
Which member of the following pairs is the stronger acid? Give reasons for your choice. (a) [Fe(OH 2 ) 6 ] 3+ or [Fe(OH 2 ) 6 ] 2+ , (b) [Al(OH 2 ) 6 ] 3+ or [Ga(OH 2 ) 6 ] 3+ , (c) Si(OH) 4 or...
-
Use Paulings rules to place the following acids in order of increasing acid strength: HNO 2 , H 2 SO 4 , HBrO 3 , and HClO 4 in a nonlevelling solvent.
-
Arrange the following ions in order of increasing acidity in aqueous solution: Fe 3+ , Na + , Mn 2+ , Ca 2+ , Al 3+ , Sr 2+ .
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


