Hydrogen possesses three isotopes, but tritium ( 3 H), which is radioactive, occurs as less than 1
Question:
Hydrogen possesses three isotopes, but tritium (3H), which is radioactive, occurs as less than 1 in 1017 atoms in a sample of natural hydrogen. If the value of Ar for hydrogen is 1.008, estimate the percentage abundance of protium, 1H, and deuterium, 2H (or D) present in a sample of natural hydrogen. Point out any assumptions that you make. Explain why your answers are not the same as those quoted in Appendix 5.
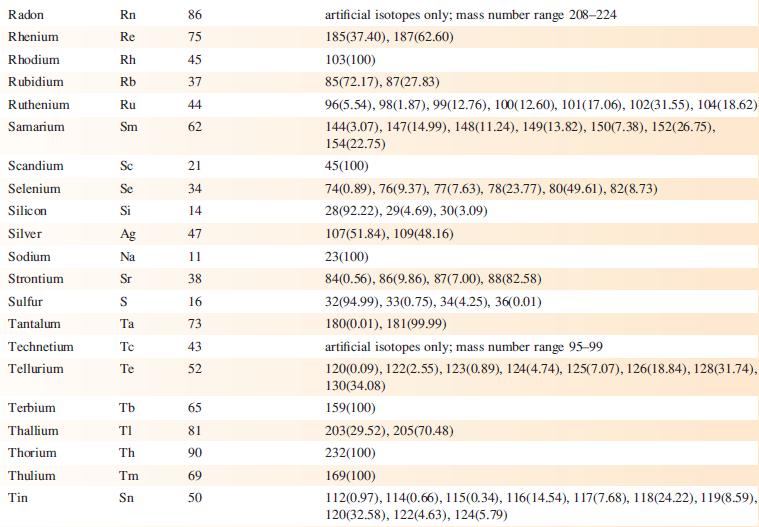
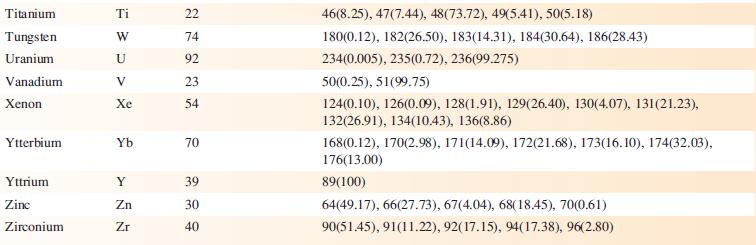
Data from Appendix 5
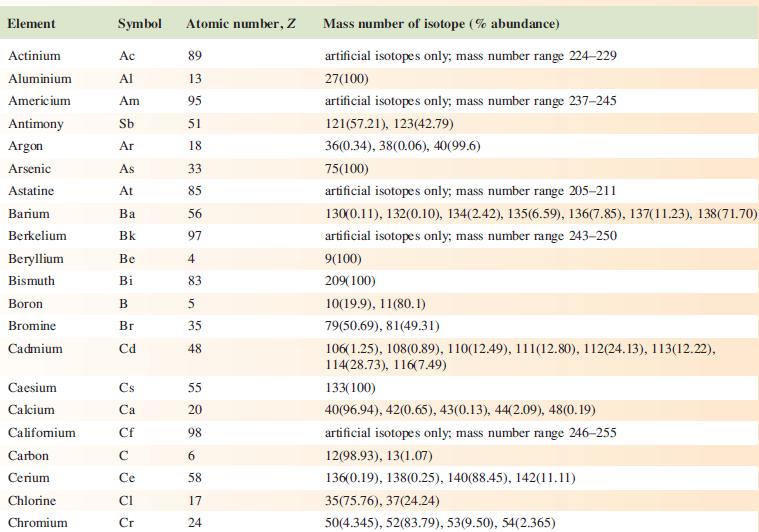
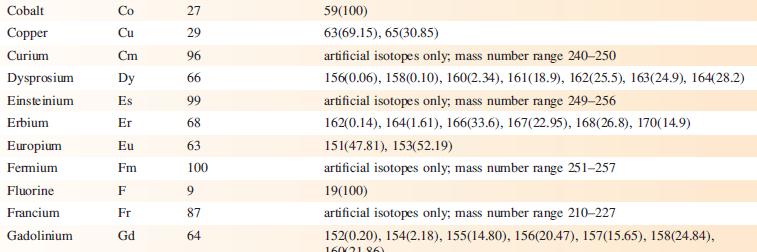
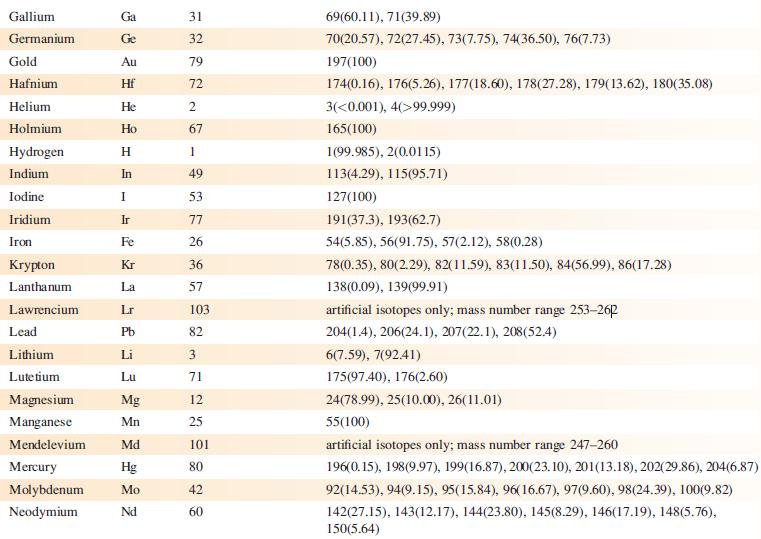
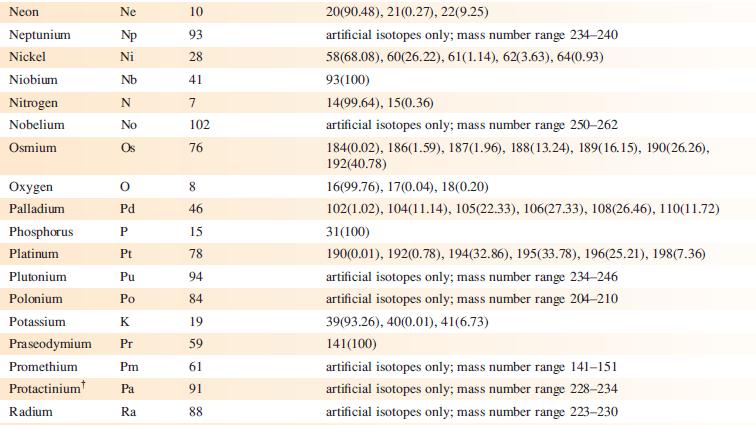
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:




