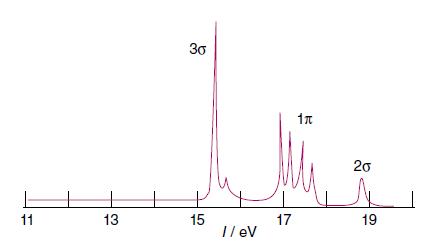
Assign the lines in the UV photoelectron spectrum of CO shown in Fig. 2.31 and predict the
Question:
Assign the lines in the UV photoelectron spectrum of CO shown in Fig. 2.31 and predict the appearance of the UV photoelectron spectrum of the SO molecule.
Figure 2.31.
Transcribed Image Text:
11 13 30 15 // eV 17 1π 20 19
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
In Fig 231 the UV photoelectron spectrum of CO is shown The photoelectron spectrum provides informat...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
The photoelectron spectrum of CO has four major peaks with ionization energies of 14.5, 17.2, 20.1, and 38.3 eV. Assign these peaks of molecular orbitals of CO, and prepare a quantitative energy...
-
The photoelectron spectrum of CO has four major peaks with ionization energies of 14.5, 17.2, 20.1, and 38.3 eV. Assign these peaks of molecular orbitals of CO, and prepare a quantitative energy...
-
The 1H NMR spectrum of 1-chloropentane is shown at 60 MHz (spectrum H) and 500 MHz (spectrum I), Explain the differences in appearance of the two spectra, and assign the signals to specific hydrogens...
-
Coca-Cola is seeking: To determine the effect of age on intention to purchase Coke Zero; To examine the effect of price on intention to purchase Coke Zero. What are the independent variable(s) and...
-
E-Eyes.com Bank just issued some new preferred stock. The issue will pay an annual dividend of $20 in perpetuity, beginning 20 years from now. If the market requires a return of 5.8 percent on this...
-
Jazzy Antiques purchased the copyright on a watercolour painting for $177,480 on January 1, 2023. The copyright legally protects its owner for 19 more years. However, Jazzy plans to market and sell...
-
A representative is selected at random. Find the probability of each event. (a) The representative is male. (b) The representative is a Republican. (c) The representative is male given that the...
-
Wagner Manufacturing estimated its product costs and volume of production for 2012 by quarter as follows. Wagner Company sells a souvenir item at various resorts across the country. Its management...
-
Unida Systems has 47 million shares outstanding trading for $12 per share. In addition, Unida has $91 million in outstanding debt. Suppose Unida's equity cost of capital is 16%, its debt cost of...
-
What are the expected changes in bond order and bond distance that accompany the following ionization processes? (a) 0 0; +e; (b) N +eN; (c) NO-NO+ + e
-
Assume that the MO diagram of IBr is analogous to that of ICl (Fig. 2.24). (a) What basis set of atomic orbitals would be used to generate the IBr molecular orbitals? (b) Calculate the bond order of...
-
Understand the decision logic of strategy development and be able to discuss its steps.
-
Q Proprietorinc (the lessee) enters into a 10 year lease of a property with an option to extend the contract for 5 years. Lease payments are $50,000 per year, payable at the beginning of each year....
-
1.Think about your investment Possibility for 3 years holding period in real investment environment? A.What could be your investment objectives? B. What amount of fund you could invest for three...
-
3- The student council normally sells 1500 school T-shirts for $12 each. This year they plan to decrease the price of the T-shirts. Based on student feedback, they know that for every $0.50 decrease...
-
2. The notation {f(x): x S} means "the set of all values that can be produced by substituting an element x of set S into f(x)." For example, the set of all odd integers can be expressed as {2k+1kZ}....
-
Implementation guidance for IFRS 2 indicates that it "accompanies, but is not part of, IFRS 2." In other words, this implementation guidance is considered mandatory. integral to the standard. not...
-
What is required for an enforceable security interest?
-
Establish identity. cos( + k) = (-1)k cos , k any integer
-
ReO 3 is a structure-prototype. Each Re(VI) centre is octahedrally sited with respect to the O 2 centres. The unit cell can be described in terms of a cubic array of Re(VI) centres, with each O 2...
-
Give explanations for the following observations. (a) Raising the temperature of a sample of -Fe from 298K to 1200K (at 1 bar pressure) results in a change of coordination number of each Fe atom from...
-
Comment on the structural and compositional implications of (a) The Fe-deficiency of iron(II) oxide, (b) The anion-excess nature of uranium(IV) oxide.
-
September 23 for $1,050 each. On December 24 , it sold one of the diamonds that was purchased on July 9 . Using the specific identification method, its ending inventory (after the December 24 sale)...
-
Madsen Motors's bonds have 13 years remaining to maturity. Interest is paid annually, they have a $1,000 par value, the coupon interest rate is 8%, and the yield to maturity is 10%. What is the...
-
Builder Products, Incorporated, uses the weighted - average method in its process costing system. It manufactures a caulking compound that goes through three processing stages prior to completion....

Study smarter with the SolutionInn App


