Assume that the MO diagram of IBr is analogous to that of ICl (Fig. 2.24). (a) What
Question:
Assume that the MO diagram of IBr is analogous to that of ICl (Fig. 2.24).
(a) What basis set of atomic orbitals would be used to generate the IBr molecular orbitals?
(b) Calculate the bond order of IBr.
Figure 2.24
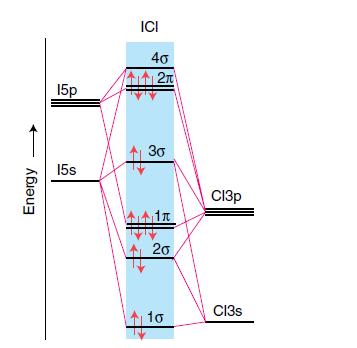
Transcribed Image Text:
Energy 150 15s ICI 40 2π 3G 1л 20 10 СІЗр Cl3s
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
a To generate the molecular orbitals MOs of IBr the basis set of atomic orbitals used would typicall...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
The iodine bromide molecule, IBr, is an inter-halogen compound. Assume that the molecular orbitals of IBr are analogous to the homo nuclear diatomic molecule F2. (a) Which valence atomic orbitals of...
-
The diatomic molecule OH exists in the gas phase. OH plays an important part in combustion reactions and is a reactive oxidizing agent in polluted air. The bond length and bond energy have been...
-
The energy-level diagram in Figure 9.36 shows that the sideways overlap of a pair of p orbitals produces two molecular orbitals, one bonding and one anti-bonding. In ethylene there is a pair of...
-
What was the action you are most proud of, or that is the most meaningful to you? What was the civic issue that was involved in your action? When and how did you first hear about that issue? Why did...
-
Feeback Corporation stock currently sells for $64 per share. The market requires a return of 11 percent on the firms stock. If the company maintains a constant 4.5 percent growth rate in dividends,...
-
At the end of its annual accounting period, Midi Company estimated its bad debts as 0.70% of its $1,700,000 of credit sales made during the year. On December 31, Midi made an addition to its...
-
A senator is selected at random. Find the probability of each event. (a) The senator is male. (b) The senator is not a Democrat. (c) The senator is female or a Republican. (d) The senator is male or...
-
a. Create a data flow diagram of the current system. b. Create a system flowchart of the existing system. c. Analyze the internal control weaknesses in the system. Model your response according to...
-
You are getting ready to start a new project that will incur some cleanup and shutdown costs when it is completed. The project costs $5.33 million up front and is expected to generate $1.17 million...
-
Assign the lines in the UV photoelectron spectrum of CO shown in Fig. 2.31 and predict the appearance of the UV photoelectron spectrum of the SO molecule. Figure 2.31. 11 13 30 15 // eV 17 1 20 19
-
Draw a molecular orbital energy-level diagram for the gaseous heteronuclear diatomic molecule boron nitride, BN. How does it differ from that for C 2 ?
-
Low interest rates may, or may not, signal that a central bank is pursuing an expansionary policy. Explain.
-
2. (3 points) NextGames Inc. has a new video game cassette for the upcoming holiday season. It is 3 trying to determine the target cost for the game if the selling price per unit will be set at $130,...
-
1. After watching the SR WEBINAR on how risk managers create better decision-making through a positive culture what do you think the three (or more) important points made during the webinar 2....
-
| Variance analysis, multiple products. The Robin's Basket operates a chain of Italian gelato stores. Although the Robin's Basket charges customers the same price for all flavors, production costs...
-
Question 31 of Your local coffee shop is extremely busy, so the cashier asks what you'd like to order and your name. The cashier writes this information onto a cup and passes it to the barista. After...
-
Find SSR = xy Rx+1 -dA, R= [0,2] x [4,4] Round your answer to four decimal places.
-
Filing for bankruptcy under Chapter 11 may involve a time-consuming process. How might this affect the likelihood that a firm will be able to negotiate a workout agreement with its creditors?
-
Use the information given about the angles and to find the exact value of: (a) sin( + ) (b) cos( + ) (c) sin( - ) (d) tan ( + ) (e) sin(2) (f) cos (2) (g) sin /2 (h) cos/2 cos = 4/5, 0 < < /2; cos =...
-
Comment on each of the following: (a) The difference between extrinsic and intrinsic defects; (b) why CaO is added to ZrO 2 used in refractory materials; (c) The formation of solid solutions of Al 2...
-
Suggest why doping NiO with Li 2 O in air (or the presence of O 2 ) leads to an increase in electrical conductivity, and comment on the dependence of this increase on the amount of lithium dopant.
-
Why are d-block metal oxides much more frequently non-stoichiometric than are non-d-block metal oxides?
-
1,600 Balance Sheet The following is a list (in random order) of KIP International Products Company's December 31, 2019, balance sheet accounts: Additional Paid-In Capital on Preferred Stock $2,000...
-
Question 3 4 pts 9 x + 3 x 9 if x 0 Find a) lim f(x), b) lim, f(x), C), lim , f(x) if they exist. 3 Edit View Insert Format Tools Table : 12pt M Paragraph B IV A2 Tv
-
Mr. Geoffrey Guo had a variety of transactions during the 2019 year. Determine the total taxable capital gains included in Mr. Guo's division B income. The transactions included: 1. On January 1,...

Study smarter with the SolutionInn App


