Birnessite, [Na,K][Mn IV Mn III ]O 4 xH 2 O, is a mineral with a layered
Question:
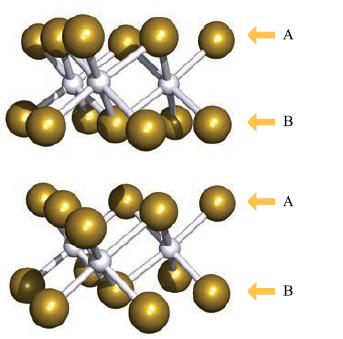
Birnessite, [Na,K][MnIVMnIII]O4 · xH2O, is a mineral with a layered structure of the same type as CdI2 (see Fig. 6.25) comprising octahedral MnO6 units. Na+ and K+ ions and H2O molecules are sited between the layers. The Na/K composition is variable. Analysis for Na is carried out using AAS. A 20 mg sample of birnessite is dissolved in concentrated HCl/HNO3 and the solution made up to 200 cm3 in a volumetric flask.
(a) Why is birnessite treated with acid?
(b) Detail how you would proceed with the sodium analysis.
(c) How would you determine the water content in the sample of birnessite?
Figure 6.25
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:




