Predict the standard enthalpies of the reactions by using mean bond enthalpy data. Assume that the unknown
Question:
Predict the standard enthalpies of the reactions
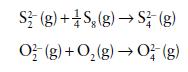
by using mean bond enthalpy data. Assume that the unknown species O42− is a singly bonded chain analogue of S42− .
Transcribed Image Text:
S2(g) +S₂(g) → S2 (g) O2(g) + O₂(g) → 0² (g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 88% (9 reviews)
a S 2 2 g 14 S 8 g S 4 2 g The enthalpy change for this reaction is zero because there is no c...View the full answer

Answered By

User l_1013947
I possess a comprehensive understanding of programming languages such as C++, Python, HTML, CSS, and Jupyter Notebook. These technical skills enable me to develop robust software solutions and create visually appealing web pages. With my expertise in coding, I can effectively tackle complex programming tasks and deliver high-quality results.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
The standard enthalpies of formation of ClO and ClO2 are 101 and 102 kJ/mol, respectively. Using these data and the thermodynamic data in Appendix C, calculate the overall enthalpy change for each...
-
`The standard enthalpies of formation of ions in aqueous solutions are obtained by arbitrarily assigning a value of zero to H+ ions; that is, Hf [H+(aq)] = 0. (a) For the following reaction ...
-
The standard enthalpies of formation of S(g), F(g), SF4(g), and SF6(g) are 1278.8 kJ/ mol, 179.0 kJ/ mol, 775 kJ/ mol, and 1209 kJ/ mol, respectively. a. Use these data to estimate the energy of an...
-
Workplace stress is sometimes related to time management. Describe a time when you were stressed at work or school because of time management. Explain how you would advise an employee or student to...
-
In the chapter, we mentioned that many companies have been under pressure to declassify their boards of directors. Why would investors want a board to be declassified? What are the advantages of a...
-
Confirm Theorem 5 for the Markov chain in Example 6. EXAMPLE 6 A Markov chain on {1, 2, 3} has transition matrix P= = 1 0 1 0 2 3 0 1 0 0 1 0 1 2 3
-
12. Certain agencies often categorize company shares as growth stocks or value stocks. What is the difference between growth and value stocks? Why are these labels misleading?
-
Recall our example of an investment of $100,000 in research that yields a pioneering invention that has no commercial value, and a subsequent investment of $50,000 in development that yields an...
-
8) Which of the following statements about internal controls is correct? n Select one: O a. a. Segregation of duties involves physically segregating employees so that they are better able to focus on...
-
Solid phosphorus pentachloride is an ionic solid composed of PCl 4 + cations and PCl 6 anions, but the vapour is molecular. What are the shapes of the ions in the solid?
-
In which of the species lCl 6 and SF 4 is the bond angle closest to that predicted by the VSEPR model?
-
Describe the relationships among the four basic financial statements and how management uses financial statements. There is a natural relationship among the four basic financial statements so that...
-
Considering your self-reflection, your personal and professional experience, and the other ideas related to leadership that you have explored in your studies so far, address the following: Provide a...
-
This research report analyzes the economics of Toyota's automobile industry between 2012-2022. The company was founded in Japan in 1937 and became one of the largest companies in the world in 2020....
-
Define workplace violence and discuss the different forms it can take. Analyze and share an example of workplace violence (maintaining confidentiality where necessary), or a hypothetical scenario....
-
Prepare a lengthy journal article on how to use learning theories (behaviorism, social cognitive, information processing, and constructivism) to improve their effectiveness as communicators of the...
-
Reflect on two to three (2-3) TV shows in which characters demonstrate aggression or violence. Consider the context in which this aggression or violence occurred and ways in which it can lead to...
-
Why must a negotiable instrument be signed?
-
What is the mode?
-
(a) The lists below show wrongly paired molecules or ions and point groups. Assign the correct point group to each species. (b) A molecule X 2 H 6 belongs to the D 3d point group. Does the molecule...
-
What is meant by a ligand group orbital?
-
In the description of the bonding of B 2 H 6 , we draw the conclusion that the two bonding MOs in Fig. 5.33 have BH bonding character delocalized over the four bridge atoms. (a) What other character...
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...
-
John has to choose between Project A and Project B, which are mutually exclusive. Project A has an initial cost of $30,000 and an internal rate of return of 16 percent. Project B has an initial cost...
-
Complete the table below, for the above transactions

Study smarter with the SolutionInn App


