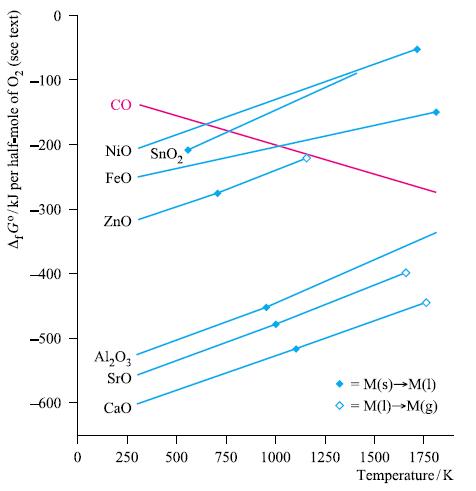
By referring to Fig. 8.6, deduce whether carbon could be used to extract Sn from SnO 2
Question:
By referring to Fig. 8.6, deduce whether carbon could be used to extract Sn from SnO2 at
(a) 500 K;
(b) 750 K;
(c) 1000 K.
Justify your answer.
Figure 8.6.
Transcribed Image Text:
A,Go/kJ per half-mole of O₂ (see text) -100 -200 -300 -400 -500 -600 0 CO NiO SnO₂ FeO ZnO Al₂O3 SrO CaO 250 500 750 M(s)→M(1) >= M(1)→→M(g) 1000 1250 = 1500 1750 Temperature/K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To deduce whether carbon could be used to extract Sn from SnO2 at different temperatures 500 K 750 K ...View the full answer

Answered By

User l_917591
As a Business Management graduate from Moi University, I had the opportunity to work as a tutor for undergraduate students in the same field. This experience allowed me to apply the theoretical knowledge I had gained in a practical setting, while also honing my teaching and communication skills.
As a tutor, I was responsible for conducting tutorial sessions, grading assignments and exams, and providing feedback and support to my students. I also assisted with the preparation of course materials and collaborated with other tutors and professors to ensure consistency in teaching and assessment.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-6. On December 12, Irene purchased the building where her store is located. She paid...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-7. Ivan sold the following securities during the year and received a Form 1099-B that...
-
Derive the velocity potential for a doublet; i.e., derive Eq. (3.88).
-
Give an example of business transactions that would: (a). Cause one asset to increase and another asset to decrease, with no effect on either liabilities or owners equity. (b). Cause both total...
-
When working on a network, create a list that will help to prioritise the tasks on time. Discuss the tasks with your team and create the list.
-
7. What is a sales invoice?
-
Jay Seago is suing the manufacturer of his car for $3.5 million because of a defect that he believes caused him to have an accident. The accident kept him out of work for a year. The company has...
-
Sunshine Sushi, a Japanese restaurant, has the following adjusted trial balance with accounts listed in alphabetical order. For the bank loan, $69,300 is due in 2021. For Notes receivable, $33,750...
-
Comment on the trends in values of (a) Melting points, (b) atom H(298 K) (c) fus H(mp) for the elements on descending group 14.
-
(a) Write down, in order, the names and symbols of the elements in group 14. (b) Classify the elements in terms of metallic, semi-metallic or non-metallic behaviour. (c) Give a general notation...
-
Suppose you own wealth w and you are facing a potential loss l that may occur with probability p. There is an insurance company that offers protection against an arbitrary loss at an actuarial fair...
-
You are required to work with your groups for the restaurant business that you have created and develop your international market entry strategy. Please follow the below steps: STEP 1: Research the...
-
Demonstrate to the owner of the business how they could use e-commerce for example (Shopify, E-Bay, Etsy), social media for example (Facebook, Instagram, TikTok, Webpage) to market their business to...
-
Identify a company that has successfully created a brand image for their product or service by using social media. Explain how they did it . Which forms of media did they use?
-
The Magic that Makes Customer Experiences Stick Article Identify and explain in details Roger's Five Factors as it applies to the diffusion process.
-
Topic: Buick in China Task: After reading and viewing the items in this week's Reading & Study folder, identify and describe the following: the social and cultural aspects that made China attractive...
-
Select a business and describe the alternative growth options available to the firm in the global environment. Which would you recommend and why?
-
Wimot Trucking Corporation uses the units-of-production depreciation method because units-of-production best measures wear and tear on the trucks. Consider these facts about one Mack truck in the...
-
In their article Targeting and delivery of platinum-based anticancer drugs (Chem. Soc. Rev. 2012, 42, 202), X. Wang and Z. Guo review the expanding field of nanoparticle-based drug delivery....
-
Compounds of Au(III) are under investigation as anticancer drugs. Predict some of the similarities and contrasts with Pt(II) compounds.
-
Reductive dehalogenases are cobalamin-containing enzymes which catalyse the replacement of a halogen atom by a hydrogen atom in organic molecules (X is typically Cl). With reference to the principles...
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


