Calculate the overall formation constant for [Fe(CN) 6 ] 3 , given that the overall formation constant
Question:
Calculate the overall formation constant for [Fe(CN)6]3−, given that the overall formation constant for [Fe(CN)6]4− is ≈ 1032, and that:
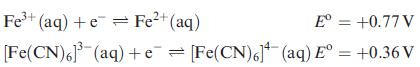
Transcribed Image Text:
Fe³+ (aq) + e Fe²+ (aq) [Fe(CN)6]³(aq) E = +0.77 V + e[Fe(CN)6] (aq) E° = +0.36 V
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
To calculate the overall formation constant for FeCN63 we can use the Nernst equation which relates ...View the full answer

Answered By

Aketch Cindy Sunday
I am a certified tutor with over two years of experience tutoring . I have a passion for helping students learn and grow, and I firmly believe that every student has the potential to be successful. I have a wide range of experience working with students of all ages and abilities, and I am confident that I can help students succeed in school.
I have experience working with students who have a wide range of abilities. I have also worked with gifted and talented students, and I am familiar with a variety of enrichment and acceleration strategies.
I am a patient and supportive tutor who is dedicated to helping my students reach their full potential. Thank you for your time and consideration.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The firm gets 70% of its capital from common stock and 30% from debt. The debtholders required rate of return is 8%. The equity holders required rate of return is 13% and the firms tax rate is 20%....
-
The overall formation constant for HgI42- is 1.0 Ã 1030. That is, [Hgl,-) [Hg 1.0 x 1030 =
-
Use the solubility-product constant for Cr(OH) 3 (K sp = 6.7 10 -31 ) and the formation constant for Cr(OH) 4 from Table 17.1 to determine the concentration of Cr(OH) 4 in a solution that is...
-
Which of the following options are available for creating a policy in Qualys Policy Compliance? (Choose three) A, Create from Host B, Create from Scratch C, Import from Library D, Import from CSV File
-
Global competition is determined in part by both efficiency and innovation. To develop a foreign market entry strategy for your company, a colleague informed you of a competitiveness report that is...
-
Recall that any directed acyclic graph G has an associated family of probability distributions, which consists of all probability distributions that can be represented by a Bayes net with structure...
-
Which growth strategies have been pursued by Starbucks and Dunkin Donuts in the past? Which strategies do you believe will be most successful for the two firms in the future? Why? THE COFFEE WARS In...
-
At the end of 2016 the following information is available for the Alaska Company and the Colorado Company: Required a. For each company, compute the debt to assets ratio and the return on equity...
-
Use the option quote information shown here to answer the questions that follow. The stock is currently selling for $68. Calls Puts Strike Option Expiration Price Vol. Last Vol. Last RWJ Mar 63 238...
-
Using data from Table 8.1, write down the spontaneous cell process, and calculate E o cell and G o for the following combinations of half-cells: Data from Table 8.1 (a) Ag+ (aq) +e=Ag(s) (b) Br (aq)...
-
Using appropriate data from eqs. 8.43 to 8.47, confirm the value of E o given for eq. 8.48. Equations 12+ (aq) + 2e Mn(s) Mn+ [MnO4] (aq) + e[MnO4) E = -1.19 V (aq) E = +0.56 V 2+ MnO (s) + 4H+ (aq)...
-
The December 31, 2012, statement of financial position for the Blood Donors of America Foundation is presented below. Statement of Financial Position December 31, 2012 Assets...
-
Analysts and investors often use return on equity ( ROE ) to compare profitability of a company with other firms in the industry. ROE is considered a very important measure, and managers strive to...
-
Provide a brief summary of the case. Respond to the following: 1. Discuss the factors which contributed to the success of the change process in terms of unfreeze, move, and refreeze stages in force...
-
Prepare a proposal where a government agency meets with consumer groups and producers on how to address the shortages in rice, sugar, onions, and fuel, i.e. oil, gasoline and the like. Use the format...
-
Decided to embark on a personal improvement project centered around time management after reviewing the insightful workbook by Neuhauser et al. (2004). My decision was influenced by my recognition...
-
You are the Senior Manager of IAuditYou LLP, you were recently assigned to take over a very important client for the company, The engagement partner, Max Roff, has been the audit partner for the past...
-
(a) Find the critical F-value for a right-tailed test with a = 0.05, degrees of freedom in the numerator = 8, and degrees of freedom in the denominator = 9. (b) Find the critical F-value for a...
-
Define cultural intelligence. Cite the books or journal articles you found in Capella's library. Explain why cultural intelligence is important for HR practitioners and other organizational managers.
-
In their review on new commercial applications of Ln chemistry (Acc. Chem. Res., 2016, 49, 844), Guillou and coworkers explain how the coordination of different Ln(III) ions within metalorganic...
-
Predict what species are formed when Pu metal is dissolved in dilute HCl and the nature of the solid product that forms when HF is subsequently added.
-
Explain why UF 3 and UF 4 are high melting point solids whereas UF 6 sublimes at 57C.
-
Break-Even Sales and Sales to Realize Income from Operations For the current year ending October 31, Yentling Company expects fixed costs of $537,600, a unit variable cost of $50, and a unit selling...
-
You buy a stock for $35 per share. One year later you receive a dividend of $3.50 per share and sell the stock for $30 per share. What is your total rate of return on this investment? What is your...
-
Filippucci Company used a budgeted indirect-cost rate for its manufacturing operations, the amount allocated ($200,000) is different from the actual amount incurred ($225,000). Ending balances in the...

Study smarter with the SolutionInn App


