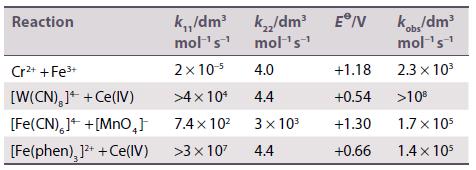
Calculate the rate constants for outer-sphere reactions from the following data. Compare your results to the measured
Question:
Calculate the rate constants for outer-sphere reactions from the following data. Compare your results to the measured values in the last column.
Transcribed Image Text:
Reaction Cr²+ + Fe³+ [W(CN),]++ Ce(IV) [Fe(CN),]+ +[MnO₂] [Fe(phen), ]²+ + Ce(IV) k.,,/dm³ mol ¹s¹ 2x 10-⁹ >4 x 10¹ 7.4×10² >3 x 10² k₂/dm³ E/V mol ¹s¹ 4.0 4.4 3× 10³ 4.4 +1.18 +0.54 +1.30 +0.66 kobs/dm³ mol ¹s¹ 2.3 × 10³ >10⁰ 1.7 x 105 1.4 x 105
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
To calculate the rate constants for outersphere reactions we can use the Marcus crossrelation equati...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
BACKGROUND You are an information analyst working for NEE. The company president has asked you to prepare a Quantitative analysis of financial, sales, and operations data to help determine which...
-
The equilibrium Ac=' B + C at 25C is subjected to a temperature jump that slightly increases the concentrations of Band C. The measured relaxation time is 3.0 us. The equilibrium constant for the...
-
Calculate the intrinsic value of Disney by conducting a two-stage dcf company-level valuation analysis, Compare your results to the current market capitalization of the company and Perform a...
-
(5 points) In a study of purchasing behavior at a small shop, it was found that the probabaty that a purchase is more than $5 is 0.29, the probability that a customer will pay with a credit card is...
-
Farley Company identifies the following items for possible inclusion in the taking of a physical inventory. Indicate whether each item should be included or excluded from the inventory taking. (a)...
-
Outline the key differences between how people learn.
-
Calculating the Present Value of a Consumer Purchase. What would be the net present value of a microwave oven that costs $159 and will save you $68 a year in time and food away from home? Assume an...
-
On January 4, 2013, Runyan Bakery paid $324 million for 10 million shares of Lavery Labeling Company common stock . The investment represents a 30% interest in the net assets of Lavery and gave...
-
Current Attempt in Progress Cullumber Company is the creator of Y-Go, a technology that weaves silver into its fabrics to kill bacteria and odor on clothing while managing heat. Y-Go has become very...
-
The activation enthalpy for the reduction of cis-[CoCl 2 (en) 2 ] + by Cr 2+ (aq) is 24 kJ mol 1 . Explain the negative value.
-
Octahedral complexes of metal centres with high oxidation numbers or of d metals of the second and third series are less labile than those of low oxidation number and d metals of the first series of...
-
The integrative social contracts view of ethics suggests that decisions should be based on religious convictions. True or False
-
Use the following data to calculate the requested ratios for Tristar Transport and Logistic Services. Briefly analyse each answer. 1) Days accounts receivable. 2) Inventory turnover. 3) Debt/equity....
-
The following partial information is contained in the variance analysis received from the Western Plant of Eastlawn Company. All plants at Eastlawn apply overhead on the basis of direct labor-hours....
-
The Hudson Company is the sponsor of an IRS qualified defined benefit pension plan for a single employer. The pension plan calculates pension benefits based on factors like age, years of service, and...
-
Find the area enclosed by one loop of the four-leaved rose r = cos(20).
-
Landen Corporation uses a job-order costing system. At the beginning of the year, the company made the following estimates: Direct labor-hours required to support estimated production 65,000...
-
Based on your Internet research in problem 1, can you answer the following? a. We described different types of venture capital funds that exist in Canada. What are the main differences in investment...
-
Suppose that fraction used = / 1.0 + 0.1Mt. for some parameter 1. Write the discrete-time dynamical system and solve for the equilibrium. Sketch a graph of the equilibrium as a function of ....
-
If the reaction Fe 2 N(s) + 3/2H 2 (g) 2Fe(s) + NH 3 (g) comes to equilibrium at a total pressure of 1 bar, analysis of the gas shows that at 700. and 800. K, PNH 3 /PH 2 = 2.165 and 1.083,...
-
Draw the structure of an alkyne that can be converted into 3-ethylpentane upon hydrogenation. Provide a systematic name for this compound.
-
Propose a mechanism for each of the following transformations: (a) (b) Na NH3 (1) ,
-
4. The risk-free rate of return is 3.78% and the market risk premium is 6.42%. What is the expected rate of return on a stock with a beta of 1.09?
-
Maddox Resources has credit sales of $ 1 8 0 , 0 0 0 yearly with credit terms of net 3 0 days, which is also the average collection period. Maddox does not offer a discount for early payment, so its...
-
Selk Steel Co., which began operations on January 4, 2017, had the following subsequent transactions and events in its long-term investments. 2017 Jan. 5 Selk purchased 50,000 shares (25% of total)...

Study smarter with the SolutionInn App


