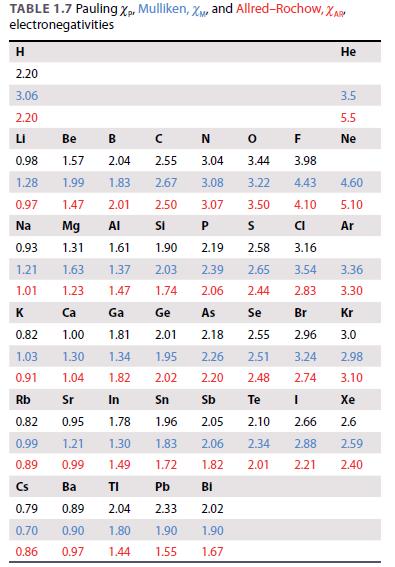
Classify the following materials using the electronegativity data in Table 1.7 and a Ketelaar triangle: (a) LiI,
Question:
Classify the following materials using the electronegativity data in Table 1.7 and a Ketelaar triangle:
(a) LiI,
(b), BeBr2,
(c) SnS,
(d) RbSn.
Table 1.7.
Transcribed Image Text:
TABLE 1.7 Pauling Xp, Mulliken, X, and Allred-Rochow, XAR electronegativities H 2.20 3.06 2.20 LI 0.98 1.28 0.97 Na 0.93 1.21 1.01 к 0.82 1.03 0.91 Rb 0.82 0.99 0.89 Cs 0.79 0.70 0.86 F 3.04 3.44 3.98 3.08 3.22 4.43 3.07 3.50 4.10 P CI 2.19 3.16 2.39 3.54 1.47 1.74 2.06 2.83 Br 2.96 3.24 2.74 Be B 1.57 2.04 2.55 1.99 1.83 2.67 1.47 2.01 2.50 Mg AI Si 1.31 CN 1.61 1.90 1.63 1.37 2.03 1.23 Ca 1.00 1.30 1.04 Sr In 0.95 1.78 1.96 1.21 1.30 1.83 0.99 1.49 1.72 Ba TI Pb 0.89 2.04 2.33 0.90 1.80 1.90 0.97 2.58 2.65 2.44 Ge As Se 2.18 2.55 2.26 2.51 2.02 2.20 2.48 Sn Sb Te [ 2.05 2.10 2.06 2.34 1.82 2.01 Ga 1.81 2.01 1.34 1.95 1.82 O Bi 2.02 1.90 1.44 1.55 1.67 s 2.66 2.88 2.21 He 3.5 5.5 Ne 4.60 5.10 Ar 3.36 3.30 Kr 3.0 2.98 3.10 Xe 2.6 2.59 2.40
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The Ketelaar triangle is a graphical representation of electronegativity values that helps us understand the polarity of bonds between elements It is ...View the full answer

Answered By

Deepankur Keserwani
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
As an operations manager, you know that you need to able to quantify the performance of your process. That means that youll need to have certain Key Performance Indicators (KPIs) in place, as well as...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Table 11.11 is from a Kansas State University survey of 262 pig farmers. For the question What are your primary sources of veterinary information?, the categories were (A) professional consultant,...
-
Under Social Security, the family of a worker who dies while fully insured at the time of death has a right to survivors' benefits. True False
-
Find the APR, or stated rate, in each of the followingcases: Stated Rate (APR) Number of Times Compounded Effective Rate (EAR) Semiannually Monthly Weekly Infinite 11.5% 12.4 10.1 13.8
-
Natalie had a very busy May. At the end of the month, after Natalie has journalized and posted her adjusting and correcting entries, she has prepared an adjusted trial balance. Instructions Using the...
-
Why should a project manager be concerned with monitoring a projects progress? AppendixLO1
-
A block (mass m1) lying on a frictionless inclined plane is connected to a mass m2 by a mass less cord passing over a pulley, as shown in Fig. 4-57. (a) Determine a formula for the acceleration of...
-
Accounts receivable at the end of the month are $1,000,000. Bad debts are expected to be 2.0% of accounts receivable. If allowance for doubtful accounts has a credit balance of $15,000 before...
-
An alloy of copper and gold has the structure shown in Fig. 4.75. Calculate the composition of this unit cell. What is the lattice type of this structure? Given that 24-carat gold is pure gold, what...
-
Determine the formulas of the compounds produced by (a) Filling a quarter of the tetrahedral holes with cations M in a hexagonal close-packed array of anions X; (b) Filling half the octahedral holes...
-
What is the resolving power of a microscope ( = 550 nm) with a 5-mm-diameter objective which has f = 9 mm?
-
Part 2 Problems 1. Jets Corp. maintains its books on a cash basis. However, the company obtained a loan of $150,000 from a local bank. The bank requires Jets Corp. to provide annual financial...
-
Company A has a well-developed brand website. They send e-mails to customers and prospects. However, the company has never used social media or mobility marketing. You have been called upon to...
-
Is Time Running Out for Bed Bath & Beyond case study and answer following questions: 3-13 analyze bed bath & beyond using the competitive forces and value chain models. 3-14 define the problem faced...
-
Marcus expresses an interest in learning more about Katie's job position, telling her that he hopes to be in the position himself one day. Katie decides to take Marcus under her wing and teach him...
-
Losing to a Weaker Foe What began as a heavily conventional military campaign to unseat the regime of Saddam Hussein had become a bitter, unconventional struggle against frustrated Sunnis who...
-
In what circumstance does undue influence occur?
-
Which property determines whether a control is available to the user during run time? a. Available b. Enabled c. Unavailable d. Disabled
-
Alkali metal cyanides, MCN, are described as pseudohalides. (a) Draw the structure of the cyanide ion, and give a description of its bonding. (b) Interpret the structure of NaCN if it possesses an...
-
Write a brief account of the uses of the alkali metals and their compounds, with reference to relevant industrial processes.
-
Write down the formulae of the following ions: (a) Superoxide; (b) Peroxide; (c) Ozonide; (d) Azide; (e) Nitride; (f) Sodide.
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...
-
John has to choose between Project A and Project B, which are mutually exclusive. Project A has an initial cost of $30,000 and an internal rate of return of 16 percent. Project B has an initial cost...
-
Complete the table below, for the above transactions

Study smarter with the SolutionInn App


