Confirm that the difference in values of (OH) and (OD) given in Table 10.2 is consistent with
Question:
Confirm that the difference in values of ν̅(O—H) and ν̅(O—D) given in Table 10.2 is consistent with the isotopic masses of H and D.
Table 10.2
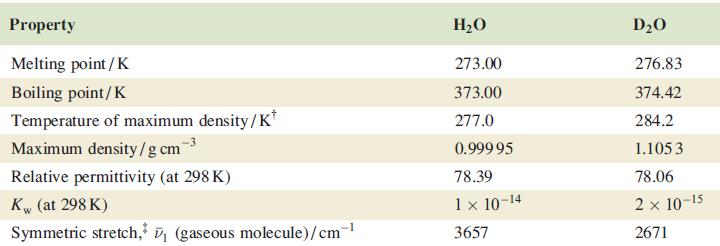
Transcribed Image Text:
Property Melting point / K Boiling point/K Temperature of maximum density/K* Maximum density/gcm Relative permittivity (at 298 K) Kw (at 298 K) Symmetric stretch, ₁ (gaseous molecule)/cm-¹ H₂O 273.00 373.00 277.0 0.999 95 78.39 1 × 10-14 3657 D₂O 276.83 374.42 284.2 1.105 3 78.06 2 x 10-15 2671
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
To confirm whether the difference in values of OH and OD given in Table 102 is consistent with the i...View the full answer

Answered By

User l_917591
As a Business Management graduate from Moi University, I had the opportunity to work as a tutor for undergraduate students in the same field. This experience allowed me to apply the theoretical knowledge I had gained in a practical setting, while also honing my teaching and communication skills.
As a tutor, I was responsible for conducting tutorial sessions, grading assignments and exams, and providing feedback and support to my students. I also assisted with the preparation of course materials and collaborated with other tutors and professors to ensure consistency in teaching and assessment.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
ABC Inc. is a U.S. manufacturing firm. It is currently in the process of issuing 20-year bonds with face value of $10,000. They expect the bond to have a credit rating of "A", and comparable A-rated...
-
In Chapter 20, we learned that to compute an approximate 90% confidence interval, the appropriate multiplier is 1.645 instead of 2.0. This works for confidence intervals for one mean or the...
-
The motivation for getting the MBA degree has many aspects-the prestige, greater opportunity for promotion, change of occupation, and an increase in pay. To focus just on this last motivation,...
-
State Newtons second law of motion. What are the limitations on the use of Newtons second law? Explain.
-
Wilmington Composites, Inc., developed its overhead application rate from the annual budget. The budget is based on an expected total output of 720,000 units requiring 3,600,000 machine hours. The...
-
Write each expression in simplest radical form. If a radical appears in the denominator, rationalize the denominator. PPv 2
-
2. Suppose you observe the prices {5, 4, 5, 6, 5}. What are the arithmetic and geometric averages? Nowyou observe {3, 4, 5, 6, 7}. What are the two averages? What happens to the difference between...
-
Homeowners Heroes Inc., manufactures household products such as windows, light fixtures, ladders, and work tables. During the year it produced 10,000 Model 10X windows but only sold 5,000 units at...
-
SCP Corporation purchased inventory on account from OBP Corporation. Which of the following happens immediately after the first step in OBPs acquisition / payment process? A. The first step in SCPs...
-
Using the information in Figs. 10.14 and 10.18, explain how the two oligonucleotides assemble into a double helical structure (see Fig. 10.14 for the 3' and 5' numbering, and definitions of C, A, G...
-
Ionic liquids show potential for applications in lithium-ion batteries. A combination of Li[N(SO 2 CF 3 ) 2 ] and [EMIm][N(SO 2 CF 3 ) 2 ] (EMim = 1-ethyl-3 methylimidazolium ion) has been used as a...
-
What is organizational culture? How does it implement strategy?
-
2 4. A rod of length 2 cm makes an angle rad with the principal axis of a thin convex lens. The lens has a focal 3 40 3 cm from the object as shown in the figure. The height of the length of 10 cm...
-
7. Consider an LC circuit, with inductance L = 0.1 H and capacitance C = 103 F, kept on a plane. The area of the circuit is 1 m. It is placed in a constant magnetic field of strength Bo which is...
-
Beach 10. The figure shows a circuit having eight resistances of 10 each, labelled R1 to R8, and two ideal batteries with voltages & = 12 V and 2 = 6 V. 1 R B1 Rs R R Which of the following...
-
12. Three plane mirrors form an equilateral triangle with each side of length L. There is a small hole at a distance /> O from one of the corners as shown in the figure. A ray of light is passed...
-
15. A small circular loop of area A and resistance R is fixed on a horizontal xy-plane with the center of the loop always on the axis of a long solenoid. The solenoid has m turns per unit length and...
-
Budgeting and forecasting - what are these two concepts and what is the importance of them to a facility owner?
-
The roof of a refrigerated truck compartment is of composite construction, consisting of a layer of foamed urethane insulation (t2 = 50 mm, ki = 0.026 W/m K sandwiched between aluminum alloy panels...
-
Discuss the industrial uses of lithium and likely future demand for compounds of the metal. How are these demands likely to be met? A useful resource is the United States Geological Survey at...
-
Under ambient conditions lithium and sodium adopt simple bcc structures. Under high pressures these alkali metals undergo a series of complex phase transitions to fcc and then lowersymmetry...
-
The auride anion, Au , has a similar ionic radius to Br at 196 pm. Predict structures for the ionic compounds CsAu and RbAu.
-
A proposed $2.5 M investment in new equipment at a 100 MG/y M&Ms factory will save the plant $800,000/y in energy costs. Assuming an annual interest rate of 5%/y (compounded annually), and an...
-
Brief Exercise 10-7 Coronado Company obtained land by issuing 2,250 shares of its $14 par value common stock. The land was recently appraised at $103,240. The common stock is actively traded at $44...
-
The following schedule reconciles Cele Co.'s pretax GAAP income Pretax GAAP income Nondeductible expense for fines Tax deductible depreciation in excess of GAAP depreciation expens Taxable rental...

Study smarter with the SolutionInn App


