Ionic liquids show potential for applications in lithium-ion batteries. A combination of Li[N(SO 2 CF 3 )
Question:
Ionic liquids show potential for applications in lithium-ion batteries. A combination of Li[N(SO2CF3)2] and [EMIm][N(SO2CF3)2] (EMim = 1-ethyl-3 methylimidazolium ion) has been used as a model for a room temperature ionic liquid electrolyte. From this mixture, crystals of Li2[EMIm][N(SO2CF3)2]3 have been isolated. The structure (X-ray diffraction) shows each Li+ ion to be in a 5-coordinate environment, bound by [N(SO2CF3)2]− ions. The latter adopt either bi- or monodentate modes and coordinate through O-donors.
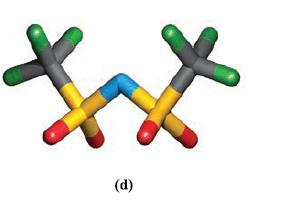
(a) When adopting a bidentate mode, why is it more favourable for a [N(SO2CF3)2]− ligand (Fig. 9.8d) to coordinate through two O atoms attached to different S atoms rather than through the two O atoms of one CF3SO2 group?
(b) What coordination geometries are usually associated with a coordination number of 5? Comment on the energy difference(s) between them.
(c) The diagrams below show the two possible arrangements of CF3 groups when [N(SO2CF3)2]− acts as an O,O'-donor to metal ion Mn+. Explain how these arise.
Figure 9.8d
Step by Step Answer:






