Answered step by step
Verified Expert Solution
Question
1 Approved Answer
6. Consider the following reaction. LOH red phosphorous Br2 R (major product) Br On estimation of bromine in 1.00 g of R using Carius

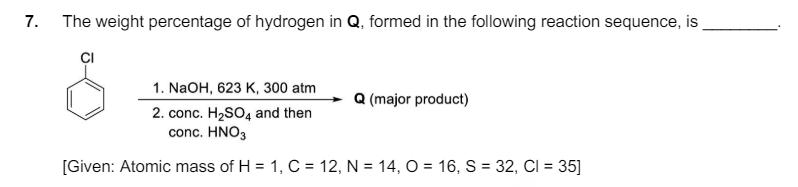
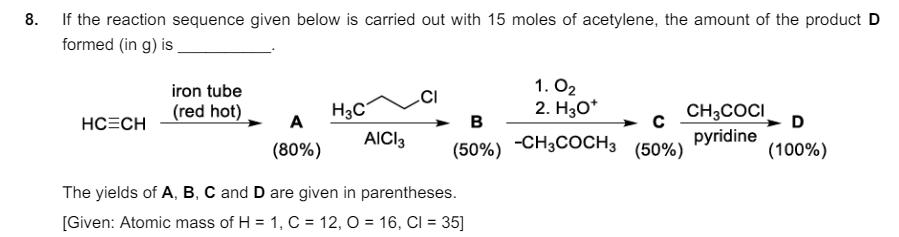
6. Consider the following reaction. LOH red phosphorous Br2 R (major product) Br On estimation of bromine in 1.00 g of R using Carius method, the amount of AgBr formed (in g) is [Given: Atomic mass of H = 1, C = 12, O= 16, P = 31, Br = 80, Ag = 108] 7. The weight percentage of hydrogen in Q, formed in the following reaction sequence, is CI 1. NaOH, 623 K, 300 atm (major product) 2. conc. H2SO4 and then conc. HNO3 [Given: Atomic mass of H = 1, C = 12, N = 14, O= 16, S = 32, CI = 35] 8. If the reaction sequence given below is carried out with 15 moles of acetylene, the amount of the product D formed (in g) is iron tube (red hot) H3C 1.02 2. H3O+ HC=CH A B C AICI 3 (80%) (50%) -CH3COCH 3 (50%) CH3COCI pyridine D (100%) The yields of A, B, C and D are given in parentheses. [Given: Atomic mass of H = 1, C = 12, O = 16, CI = 35]
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


