Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. A solution is prepared by mixing 0.01 mol each of H2CO3, NaHCO3, Na2CO3, and NaOH in 100 mL of water. pH of the
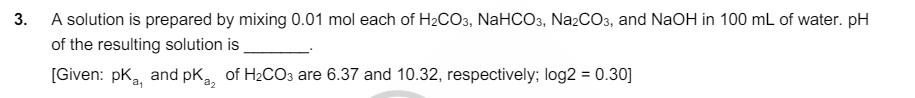
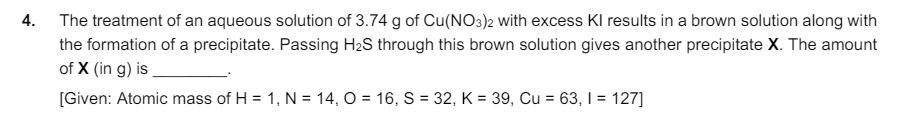
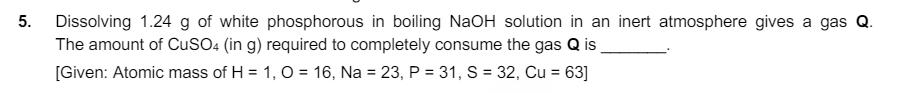
3. A solution is prepared by mixing 0.01 mol each of H2CO3, NaHCO3, Na2CO3, and NaOH in 100 mL of water. pH of the resulting solution is [Given: pk, and pKa2 of H2CO3 are 6.37 and 10.32, respectively; log2 = 0.30] 4. The treatment of an aqueous solution of 3.74 g of Cu(NO3)2 with excess Kl results in a brown solution along with the formation of a precipitate. Passing H2S through this brown solution gives another precipitate X. The amount of X (in g) is [Given: Atomic mass of H = 1, N = 14, O=16, S = 32, K = 39, Cu = 63, 1 = 127] 5. Dissolving 1.24 g of white phosphorous in boiling NaOH solution in an inert atmosphere gives a gas Q. The amount of CuSO4 (in g) required to completely consume the gas Q is [Given: Atomic mass of H = 1, O = 16, Na = 23, P = 31, S = 32, Cu = 63]
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


