17. List I describes thermodynamic processes in four different systems. List II gives the magnitudes (either exactly or as a close approximation) of possible
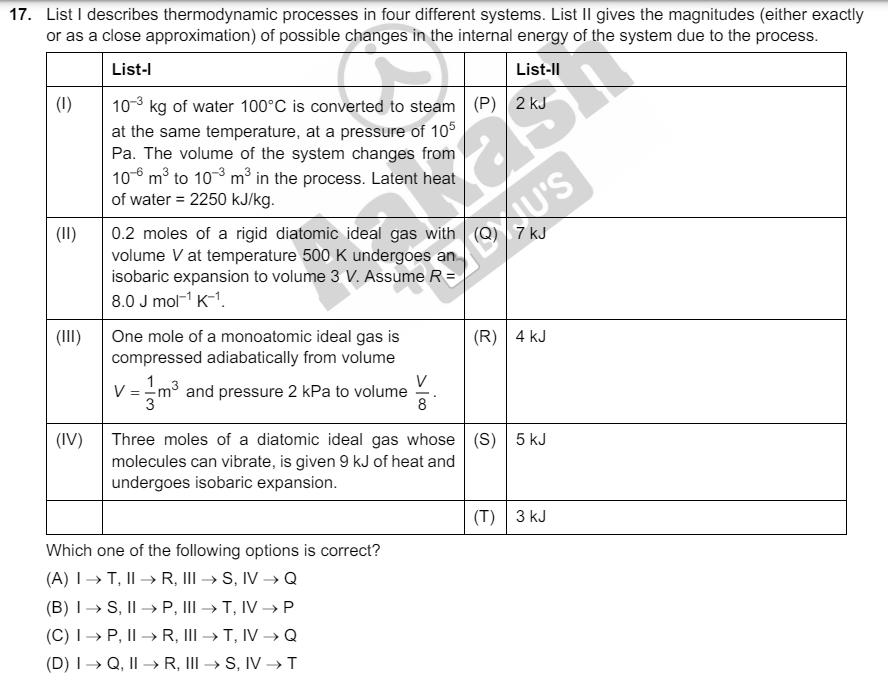
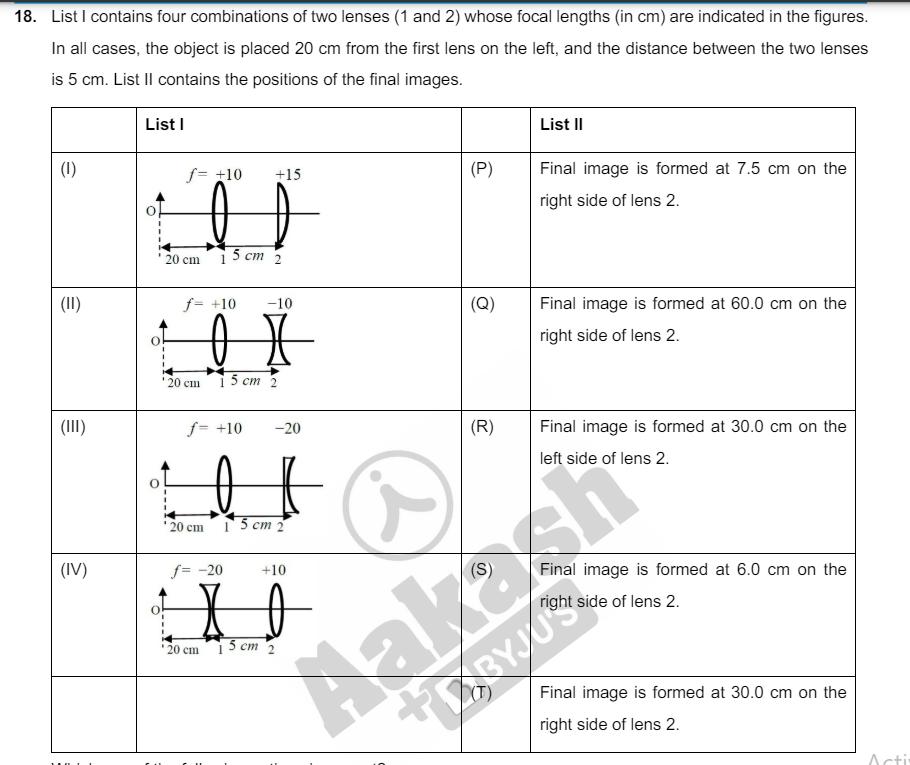

17. List I describes thermodynamic processes in four different systems. List II gives the magnitudes (either exactly or as a close approximation) of possible changes in the internal energy of the system due to the process. List-l List-ll (1) 103 kg of water 100C is converted to steam (P) 2 kJ at the same temperature, at a pressure of 105 (II) (III) (IV) Pa. The volume of the system changes from 3 106 m to 103 m in the process. Latent heat of water = 2250 kJ/kg. VOXU'S 0.2 moles of a rigid diatomic ideal gas with (Q) 7 kJ volume V at temperature 500 K undergoes an isobaric expansion to volume 3 V. Assume R = 8.0 J mol K-1 One mole of a monoatomic ideal gas is compressed adiabatically from volume V = 1 m and pressure 2 kPa to volume (R) 4 kJ V 8 Three moles of a diatomic ideal gas whose (S) 5 kJ molecules can vibrate, is given 9 kJ of heat and undergoes isobaric expansion. (T) 3 kJ Which one of the following options is correct? (A) IT, II R, III S, IV Q (B) IS, IIP, III T, IV P (C) IP, IR, III T, IV Q (D) IQ, IIR, III S, IV T 18. List I contains four combinations of two lenses (1 and 2) whose focal lengths (in cm) are indicated in the figures. In all cases, the object is placed 20 cm from the first lens on the left, and the distance between the two lenses is 5 cm. List II contains the positions of the final images. List I (1) (II) f= +10 +15 20 cm List II (P) Final image is formed at 7.5 cm on the right side of lens 2. 15 cm 2 f= +10 -10 (Q) Final image is formed at 60.0 cm on the right side of lens 2. (III) I 20 cm 15 cm 2 (IV) f= +10 -20 (R) Final image is formed at 30.0 cm on the 20 cm f= -20 15 cm 2 +10 (S) 20 cm 15 cm 2 left side of lens 2. Final image is formed at 6.0 cm on the right side of lens 2. TRYJU Final image is formed at 30.0 cm on the right side of lens 2. Acti Which one of the following options is correct? (A) (1) P; (II) R; (III) Q; (IV) T (B) (1) Q; (II)P; (III) T; (IV) S (C) (1) P; (II) T; (III) R; (IV) Q (D) (I) T; (II) S; (III) Q; (IV) R
Step by Step Solution
There are 3 Steps involved in it
Step: 1

See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


