Determine G o (298 K) for the reaction: given the following data: What does the value of
Question:
Determine ΔGo(298 K) for the reaction:
![]()
given the following data:
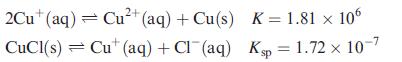
What does the value of ΔGo tell you about the tendency of precipitated CuCl to disproportionate?
Transcribed Image Text:
2+ 2CuCl(s) Cu²+ (aq) + 2Cl(aq) + Cu(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
To determine Go standard Gibbs free energy change for the given reaction we can use the relationship ...View the full answer

Answered By

Surendar Kumaradevan
I have worked with both teachers and students to offer specialized help with everything from grammar and vocabulary to challenging problem-solving in a range of academic disciplines. For each student's specific needs, I can offer explanations, examples, and practice tasks that will help them better understand complex ideas and develop their skills.
I employ a range of techniques and resources in my engaged, interesting tutoring sessions to keep students motivated and on task. I have the tools necessary to offer students the support and direction they require in order to achieve, whether they need assistance with their homework, test preparation, or simply want to hone their skills in a particular subject area.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What does the example of WorldCom tell you about the value of financial securities from different perspectives (Profit, Cash Flow, and Strategic Perspectives) as it relates to managerial decision...
-
Johnson Filtration, Inc., provides maintenance service for water filtration systems throughout southern Florida. Customers contact Johnson with requests for maintenance service on their water...
-
What does Descartes' rule of signs tell you about the number of positive real zeros and number of negative real zeros of the following function? g(x) = - x8 + 2x6 - 4x3 - 1
-
A restaurant records the following data over a month for its food: Opening inventory: $31,000 Purchases: $88,000 Closing Inventory: $28,000 Transfers in: $800 Transfers out: $200 Employee meals:...
-
How does a large-scale entry differ from a small-scale entry?
-
In Exercises use the Root Test to determine the convergence or divergence of the series. D n=0 -
-
1. What are the characteristics of a not-for-profit entity?
-
If you were trying to assess the financial health of a government administered pension plan, which financial statements or schedules would you review and why?
-
How can bank traders use front running as advantage of foreign exchange market.
-
Give the oxidation state of each element in the following compounds and ions; Pauling electronegativity values in Appendix 7 may be useful: (a) CaO; (b) H 2 O; (c) HF; (d) FeCl 2 ; (e) XeF 6 ; (f)...
-
Using data from Table 8.1 and from Section 8.3, explain why H 2 is evolved when powdered Ag is heated with a concentrated solution of HI. Data from Table 8.1 Table 8.1 Selected standard reduction...
-
Dene the degrees of freedom for the chi-square test for goodness of t and locate the critical value for a specic alpha level in the chi-square distribution.
-
Management is what tradition used to call a liberal art: "liberal" because it deals with the fundamentals of knowledge, self-knowledge, wisdom, and leadership; "art" because it is a practice and...
-
Draft a five hundred and twenty five- to seven hundred-word internal communication planthat appropriately details your proposed solution to the internal team at CVS PHARMACY. In your communication...
-
Christopher Awnings was founded by Christopher Aminim in the early days of the retirement boom in the Okanagan to build and install custom retractable awnings for retirees to keep the sun out of the...
-
Leaders are responsible for making decisions that have long-term ramifications; thus, making the appropriate decisions can be stressful and leaders' decisions may vary. They often enhance employee...
-
Employee longevity A large insurance company has developed a model to identify the factors associated with employee turnover. The dependent variable is number of years an employee stays with the...
-
Using the data from Problem 9, test whether the standard deviation of wait time at Wendy's is more than that at McDonald's at the a = 0.05 level of significance.
-
Suppose that a business sells 6-month subscriptions to its monthly magazine. On January 1, the company receives a total of $600 for 10 subscriptions. To record this transaction, the company debits...
-
From a knowledge of their chemical properties, speculate on why cerium and europium were the easiest lanthanoids to isolate before the development of ion-exchange chromatography.
-
Neither lanthanoid nor actinoid organometallic compounds obey the 18-electron rule. Discuss the reasons, using the structures of the tris(Cp) and tris(Cp*) Ln and An complexes as examples.
-
Explain the variation in ionic radii between La 3+ and Lu 3+ .
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


