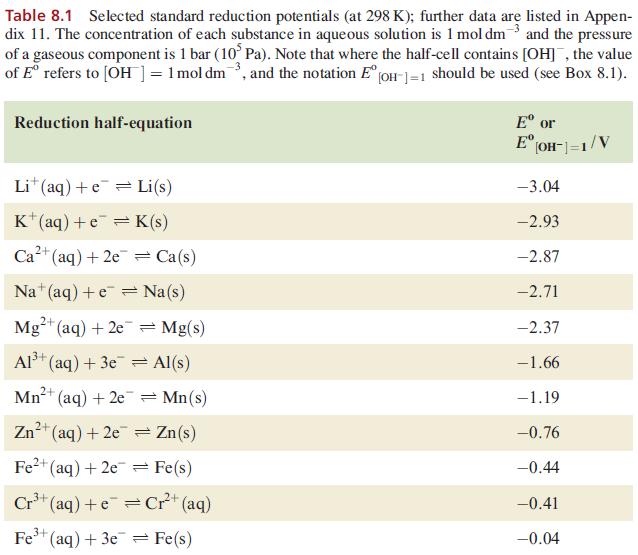
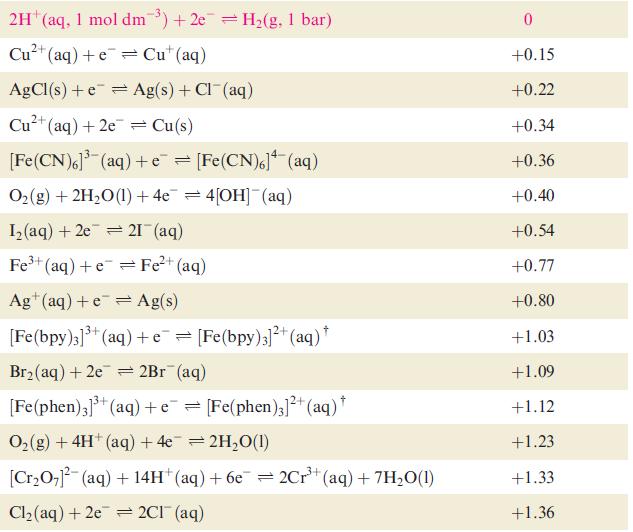
Using data from Table 8.1 and from Section 8.3, explain why H 2 is evolved when powdered
Question:
Using data from Table 8.1 and from Section 8.3, explain why H2 is evolved when powdered Ag is heated with a concentrated solution of HI.
Data from Table 8.1
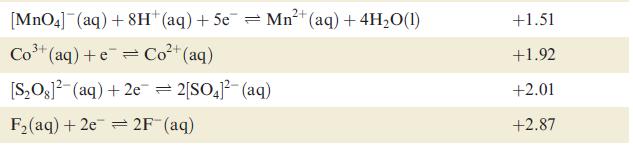
Transcribed Image Text:
Table 8.1 Selected standard reduction potentials (at 298 K); further data are listed in Appen- dix 11. The concentration of each substance in aqueous solution is 1 mol dm3 and the pressure of a gaseous component is 1 bar (10 Pa). Note that where the half-cell contains [OH], the value of Eº refers to [OH] = 1 mol dm³, and the notation Eº [OH-]=1 should be used (see Box 8.1). Reduction half-equation Lit (aq) +eLi(s) K+ (aq) + eK(s) 2+ Ca²+ (aq) +2e= Ca(s) Na (aq) +eNa(s) 2+ Mg²+ (aq) + 2e 3+ Al³+ (aq) + 3e 2+ Mn²+ (aq) + 2e 1²+ (aq) + 2e Fe²+ (aq) + 2e 3+ Cr³+ (aq) + Zn 3+ Fe³+ (aq) + 3e = Mg(s) Al(s) = Mn(s) Zn(s) Fe(s) Cr²+ (aq) = Fe(s) Eº or E [OH-]=1/V -3.04 -2.93 -2.87 -2.71 -2.37 -1.66 -1.19 -0.76 -0.44 -0.41 -0.04
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 84% (13 reviews)
To understand why H2 is evolved when powdered Ag silver is heated with a concentrated solution of HI hydrogen iodide we need to consider the redox rea...View the full answer

Answered By

User l_917591
As a Business Management graduate from Moi University, I had the opportunity to work as a tutor for undergraduate students in the same field. This experience allowed me to apply the theoretical knowledge I had gained in a practical setting, while also honing my teaching and communication skills.
As a tutor, I was responsible for conducting tutorial sessions, grading assignments and exams, and providing feedback and support to my students. I also assisted with the preparation of course materials and collaborated with other tutors and professors to ensure consistency in teaching and assessment.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following are the financial statements of Swifty Corporation. Swifty Corporation Comparative Balance Sheets December 31 Assets 2019 2018 Cash $37,200 $19,700 Accounts receivable 33,000 18,400...
-
Using data from Table 165 on page 426, assume you purchase a December 1100 (strike price) S&P 500 put option. Compute your total dollar profit or loss if the index has the following values at...
-
Using data from Table 8.4 on bond enthalpies, show that the more C---H bonds a molecule has compared to C-----O and O----H bonds, the more energy it can store.
-
Visit any social media site with news stories that includes a photograph/snapshot of story (mandatory) . Choose ONE news story. Use the questions below to help you determine validity of that ONE news...
-
Summarize the advantages of being a first mover.
-
In Exercises use the Root Test to determine the convergence or divergence of the series. n=1 (n!)" (n^)
-
2. How many categories are there for not-for-profit entities? Differentiate between their accounting and financial reporting methods.
-
What are some of the problems related to output that the committee is experiencing? What suggestions do you have for improving output to the committee? How can the budget constraints of the zoo be...
-
AAA kicks off a sales promotion on August 31, 2019. AAA included a redeemable coupon on each can of soup sold. Five coupons must be presented to receive a premium that costs AAA $2 each. AAA...
-
Determine G o (298 K) for the reaction: given the following data: What does the value of G o tell you about the tendency of precipitated CuCl to disproportionate? 2+ 2CuCl(s) Cu+ (aq) + 2Cl(aq) +...
-
Write balanced half-equations corresponding to the steps shown in the potential diagrams in Fig. 8.2. Figure 8.2 Acidic solution (pH 0) [MnO4] Alkaline solution (pH 14) [MnO] +0.90 +0.56 [HMnO4]-...
-
Define each of the following terms: a. Lessee; lessor b. Operating lease; financial lease; sale and leaseback; combination lease; synthetic lease; SPE c. Off-balance sheet financing; capitalizing d....
-
From the perspective of organizational structure, design, and control, what have gone wrong at PNB? What factors have contributed to its current state of disarray? What should be done next to help...
-
Your goal is to advise the President on domestic economic policy. Role You are the chair of the Council of Economic Advisors (CEA) Audience Your audience is the President of the United States....
-
omework quiz 2.1 #1 stem plot The miles per gallon rating for 30 cars are shown below (lowest to highest). 19, 19, 19, 20, 21, 21, 25, 25, 25, 26, 26, 28, 29, 31, 31, 32, 32, 33, 34, 35, 36, 37, 37,...
-
Your answers are saved automatically. Question Completion Status: QUESTION 1 13 points Save Answer Library A computer memory manufacturer specifies that its memory chip stores data incorrectly an...
-
Analyze the possible reasons for and responses to Chung's request for a private office. What factors might impact Leary's decision? Identify at least two challenges and dilemmas in managing...
-
If n1 = 61, s1 = 18.3, n2 = 57, and s2 = 13.5, test whether the population standard deviations differ at the = 0.05 level of significance. Perform the appropriate hypothesis test.
-
Express mass density in kg/m3 and weight density in lb/ft3. 1. Find the mass density of a chunk of rock of mass 215 g that displaces a volume of 75.0 cm3 of water. 2. A block of wood is 55.9 in. x...
-
Lanthanoid coordination compounds rarely exhibit isomerism in solution. Suggest two factors that might cause this phenomenon, explaining your reasoning. (See D. Parker, R.S. Dickins, H. Puschmann, C....
-
Sketch the a 1 symmetry-adapted orbitals for two eclipsed C 5 H 5 ligands stacked together with D 5h symmetry. Identify the s, p, and d orbitals of a metal atom lying between the rings that may have...
-
The compound [Ni( 5 -C 5 H 5 ) 2 ] readily adds one molecule of HF to yield [Ni( 5 -C 5 H 5 )( 4 -C 5 H 6 )] + , whereas [Fe( 5 -C 5 H 5 ) 2 ] reacts with strong acid to yield [Fe( 5 -C 5 H 5 ) 2 H]...
-
explain the concept of Time Value of Money and provide and example. In addition to your discussion, please explain the differences between Stocks and Bonds
-
Wildhorse Inc. has just paid a dividend of $3.80. An analyst forecasts annual dividend growth of 9 percent for the next five years; then dividends will decrease by 1 percent per year in perpetuity....
-
Jenny wanted to donate to her alma mater to set up a fund for student scholarships. If she would like to fund an annual scholarship in the amount of $6,000 and her donation can earn 5% interest per...

Study smarter with the SolutionInn App


