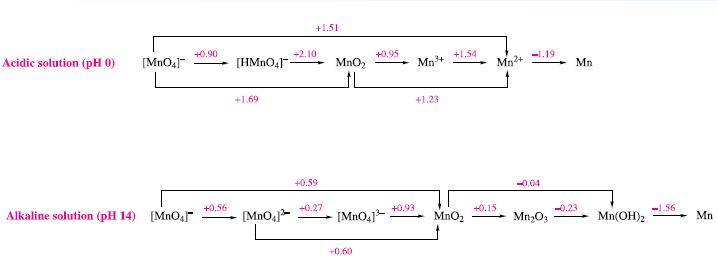
Write balanced half-equations corresponding to the steps shown in the potential diagrams in Fig. 8.2. Figure 8.2
Question:
Write balanced half-equations corresponding to the steps shown in the potential diagrams in Fig. 8.2.
Figure 8.2
Transcribed Image Text:
Acidic solution (pH 0) [MnO4] Alkaline solution (pH 14) [MnO₂] +0.90 +0.56 [HMnO4]- +1.69 [MnO4] +1.51 +2.10 +0.59 +0.27 MnO₂ L +0.95 [MnO4]³ +0,60 +0.93 Mn³+ +1.23 +1.54 MnO₂ Mn²+ +0.15 -1.19 -0.04 Mn₂O3 Mn -0.23 Mn(OH)₂ -1.56 Mn
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
To write balanced halfequations corresponding to the steps shown in the potential diagrams we need t...View the full answer

Answered By

Akash M Rathod
I have been utilized by educators and students alike to provide individualized assistance with everything from grammar and vocabulary to complex problem-solving in various academic subjects. I can provide explanations, examples, and practice exercises tailored to each student's individual needs, helping them to grasp difficult concepts and improve their skills.
My tutoring sessions are interactive and engaging, utilizing a variety of tools and resources to keep learners motivated and focused. Whether a student needs help with homework, test preparation, or simply wants to improve their skills in a particular subject area, I am equipped to provide the support and guidance they need to succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In Gruber's (1970) study of n = 104 individuals (discussed in Problem 10), the relationship between blood pressure change (SBPSL) and relative weight (RW), controlling for initial blood pressure...
-
Write equations corresponding to the following energy terms. a. The fourth ionization energy of Se b. The electron affinity of S- c. The electron affinity of Fe3+ d. The ionization energy of Mg e....
-
Write the system of linear equations corresponding to the matrix. 1. 2. 3. 4. -2 -4] -5 3
-
A certain radioactive isotope is a by - product of some nuclear reactors. Due to an explosion, a nuclear reactor experiences a massive leak of this radioactive isotope. Fortunately, the isotope has a...
-
Describe how four strategic goals may affect the decision of where to enter.
-
In Exercises determine the convergence or divergence of the series using any appropriate test from this chapter. Identify the test used. n=1 2 3
-
3. Explain how net assets, revenues, expenses, gains, and losses are classified and reported on a not-for-profit financial statement.
-
Emerson St. Paul Book Shops accounts at June 30, 2015, included the following unadjusted balances: Merchandise Inventory .........$ 5,400 Cost of Goods Sold ......... 40,300 Sales Revenue ..............
-
assuming a person short sells 300 shares of stocks, which is currently at $75 per share. what then is the maximum possible loss?
-
Using data from Table 8.1 and from Section 8.3, explain why H 2 is evolved when powdered Ag is heated with a concentrated solution of HI. Data from Table 8.1 Table 8.1 Selected standard reduction...
-
(a) Using appropriate data from Appendix 11, determine E cell for the disproportionation of H 2 O 2 . (b) Calculate G o for this process. (c) Comment on the fact that H 2 O 2 can be stored without...
-
Compute the specified ratios using Faustin Companys balance sheet for 2014. Assets Cash .............. $ 18,000 Marketable securities ......... 12,000 Accounts receivable ........ 25,000 Inventory...
-
Investigate the Mercedes Benz company and you have to cover this topic " For Business prospects, Market growth, Market quality, and Environmental aspects are three most important factors. Explain...
-
The case study for Goodwill Industries and how they "do good" as a core business strategy. What are Goodwill's competitive advantages? Goodwill has found success in social services. What problems...
-
Cosmic Cals (Pty) Ltd , a seller of personalized scientific calculators, had an inventory of 40 calculators. The value of these calculators is R15 400 each on the 1 January 2022. During the current...
-
Perform an analysis of Best Buy Co. Inc. Your analysis will draw on the Form 10K (as of February 2013). Your analysis can include information prior to February 2013 but should not draw on any...
-
Research organizational structure of a company of your choice. Use your understanding of organizational structure to analyze whether this organization's structure is the best choice for the business...
-
It has long been a concern that there is a wage gap between men and women in the United States with some reports suggesting that women only make $0.77 for every dollar earned by a man. Design a study...
-
The first national bank pays a 4% interest rate compound continuously. The effective annual rate paid by the bank is __________. a. 4.16% b. 4.20% c. 4.08% d. 4.12%
-
Suggest two plausible routes by which a carbonyl ligand in [Mo(Cp)(CO) 3 Me] might exchange for a phosphine. Neither route should invoke the initial dissociation of a CO.
-
(a) What cluster valence electron (CVE) count is characteristic of octahedral and trigonal prismatic complexes? (b) Can these CVE values be derived from the 18-electron rule? (c) Determine the...
-
Ligand substitution reactions on metal clusters are often found to occur by associative mechanisms, and it is postulated that these occur by initial breaking of an MM bond, thereby providing an open...
-
A company manufactures lawnmowers. Compute the total amount of period costs from thr following costs.
-
TestAnswerSavedHelp opens in a new windowSave & ExitSubmit Item 1 7 1 0 points Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1 : 2 0 : 1 8 Item 1 7 Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1...
-
Use the following information for the Problems below. (Algo) [The following information applies to the questions displayed below.] Lansing Company's current-year income statement and selected balance...

Study smarter with the SolutionInn App


