(a) Using appropriate data from Appendix 11, determine E cell for the disproportionation of H 2 O...
Question:
(a) Using appropriate data from Appendix 11, determine E°cell for the disproportionation of H2O2.
(b) Calculate ΔGo for this process.
(c) Comment on the fact that H2O2 can be stored without significant decomposition, unless, for example, traces of MnO2, [OH]‾ or iron metal are added.
Data from Appendix 11
The concentration of each aqueous solution is 1 mol dm−3 and the pressure of a gaseous component is 1 bar (105 Pa). (Changing the standard pressure to 1 atm (101 300 Pa) makes no difference to the values of E° at this level of accuracy.) Each half-cell listed contains the specified solution species at a concentration of 1 mol dm−3; where the half-cell contains [OH]−, the value of E° refers to [OH−] = 1 mol dm−3, hence the notation E°[OH−] = 1
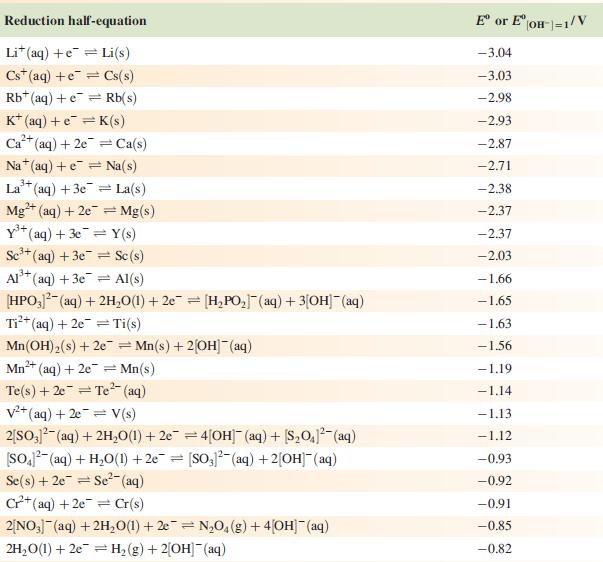

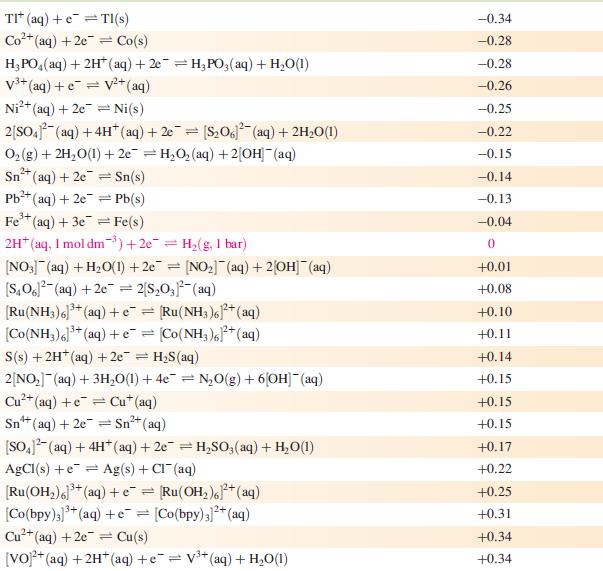
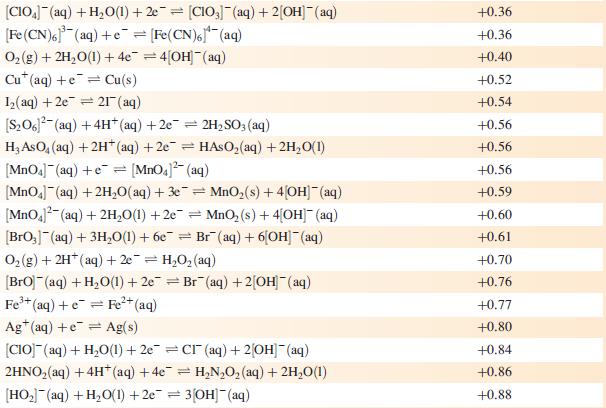
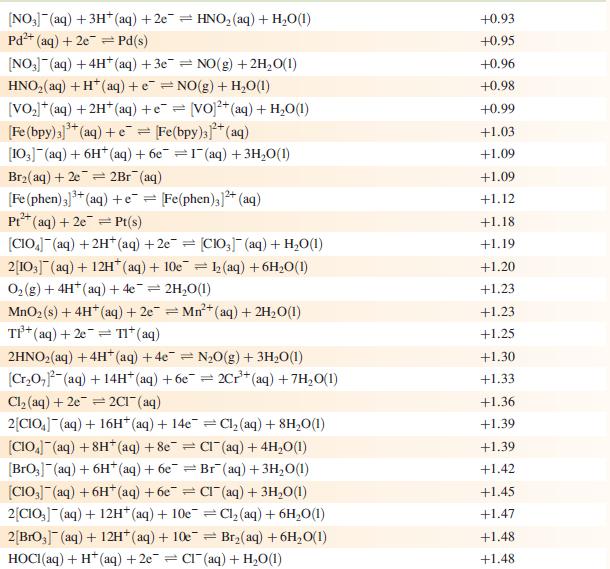

Step by Step Answer:






