Discuss the trends in stabilities of oxidation states for Group 16 elements at pH = 0, as
Question:
Discuss the trends in stabilities of oxidation states for Group 16 elements at pH = 0, as represented by the Frost diagram in Fig. 16.5, and state the likely nature of the species prevailing for each entry.
Figure 16.5.
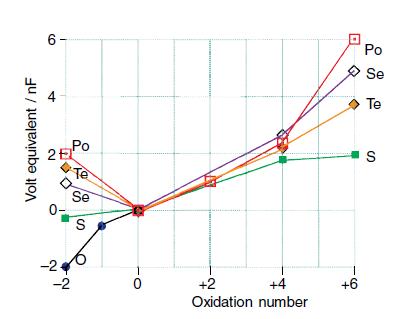
Transcribed Image Text:
Volt equivalent / nF 6- 25 Po E -2 Te Se S 1 0 +2 +4 Oxidation number +6 Po Se Te S
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
Trends in Stabilities of Oxidation States at pH 0 Oxygen O Oxygen is highly electronegative and has a strong tendency to gain electrons making the hig...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
The structures of another class of high-temperature ceramic superconductors are shown below. a. Determine the formula of each of these four superconductors. b. One of the structural features that...
-
1. How strong are the competitive forces confronting J. Crew in the market for specialty retail? Do a [Michael Porter] five-forces analysis to support your answer. (see chapter 3 in the textfor...
-
The Gap Inc. is a global specialty retailer operating stores selling casual apparel, personal care, and other accessories for men, women, and children under The Gap, Banana Republic, and Old Navy...
-
The Bay City Parks and Recreation Department is considering building several new facilities, including a gym, an athletic field, a tennis pavilion, and a pool. It will base its decision on which...
-
Harmony Industries Inc. is a small manufacturer of electronic musical instruments. The plant manager received the following variable factory overhead report for the period: The plant manager is not...
-
What are open, closed, and short circuits?
-
13. For the lookback call: a. What is the value of a lookback call as St approaches zero? Verify that the formula gives you the same answer. b. Verify that at maturity the value of the call is ST ST...
-
Wyco Park, a public camping ground near the Four Corners National Recreation Area, has compiled the following financial information as of December 31, 2019. Instructions (a) Determine Wyco Park's net...
-
You purchase a home for $207,000 that you expect to appreciate 8% in value on an annual basis. How much will the home be worth in ten years? Factors to use for n=10, 1 =8% (DO NOT USE ANY OTHER...
-
A mechanistic study of reaction between chloramine and sulfite has been reported (B.S. Yiin, D.M. Walker, and D.W. Margerum, Inorg. Chem., 1987, 26, 3435). Summarize the observed rate law and the...
-
In November 2006 the former KGB agent Alexander Litvinenko was found to have been poisoned by radioactive polonium-210. Write a review of the chemical and radiological properties of Po and discuss...
-
A clothing company is planning its winter pricing. One popular line of quarter-zip sweatshirts sells for $98. The products hit the stores at the beginning of September and sell through Christmas...
-
Lucy is using a one-sample test based on a simple random sample of size = 24 to test the null hypothesis = 23.000 cm against the alternative hypothesis < 23.000 cm. The sample has mean 22.917 cm and...
-
A motorcyclist of mass 60 kg rides a bike of mass 40 kg. As she sets off from the lights, the forward force on the bike is 200N. Assuming the resultant force on the bike remains constant, calculate...
-
A load downward load P = 400 N is applied at B. It is supported by two truss members with member BA at an angle of 0 = 45 from horizontal and member BC at an 01 = angle of 02 25 from vertical....
-
Gross profit, defined as Net sales less Cost of products sold increased by $279 million in 2017 from 2016 and decreased by $2 million in 2016 from 2015. As a percent of sales, gross profit was 38.8%...
-
An electro-magnetic shield is to be made of galvanized steel with conductivity = 1.74 x 106 S/m, and magnetic permeability HR = 80. The thickness of cold rolled steel is in the following table. Gauge...
-
1. Visit a museum or gallery exhibition or attend a theater or musical performance. The activity (museum or performance) should have fun doing this. 2. Write a report that describes your experience....
-
A police officer pulls you over and asks to search your vehicle because he suspects you have illegal drugs inside your car. Since he doesn't have reasonable suspicion to search your car, legally he...
-
In the complex [Ti(BH 4 ) 3 (MeOCH 2 CH 2 OMe)], the Ti(III) centre is 8-coordinate. Suggest modes of coordination for the ligands.
-
Comment on the variation in oxidation states of the first row metals.
-
Write out, in sequence, the first row d-block elements and give the valence electronic configuration of each metal and of its M 2+ ion.
-
7 . 4 3 Buy - side vs . sell - side analysts' earnings forecasts. Refer to the Financial Analysts Journal ( July / August 2 0 0 8 ) study of earnings forecasts of buy - side and sell - side analysts,...
-
Bond P is a premium bond with a coupon of 8.6 percent , a YTM of 7.35 percent, and 15 years to maturity. Bond D is a discount bond with a coupon of 8.6 percent, a YTM of 10.35 percent, and also 15...
-
QUESTION 2 (25 MARKS) The draft financial statements of Sirius Bhd, Vega Bhd, Rigel Bhd and Capella for the year ended 31 December 2018 are as follows: Statement of Profit or Loss for the year ended...

Study smarter with the SolutionInn App


