Draw a Frost diagram for mercury in acid solution, given the following Latimer diagram: Comment on the
Question:
Draw a Frost diagram for mercury in acid solution, given the following Latimer diagram:
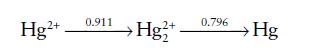
Comment on the tendency of any of the species to act as an oxidizing agent, a reducing agent, or to undergo disproportionation.
Transcribed Image Text:
Hg2+ 0.911 2+ Hg²+ 0.796 Hg
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
A Frost diagram is a graphical representation of the standard reduction potentials of various oxidat...View the full answer

Answered By

Chandrasekhar Karri
I have tutored students in accounting at the high school and college levels. I have developed strong teaching methods, which allow me to effectively explain complex accounting concepts to students. Additionally, I am committed to helping students reach their academic goals and providing them with the necessary tools to succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
Construct a Frost diagram for mercury in acidic solution. Comment on the tendency for [Hg 2 ] 2+ to disproportionate.
-
The Latimer diagram for vanadium species in acidic (pH = 0) solution is: Using these data: (a) Calculate the potential for the reduction of VO 2+ (aq) to V(s) and write a balanced chemical equation...
-
Hydrogen peroxide can function either as an oxidizing agent or as a reducing agent. At standard conditions, is H 2 O 2 a better oxidizing agent or reducing agent? Explain.
-
A vertical force P is applied to the ends of cord AB of length a and spring AC. If the spring has an unstretched length δ, determine the angle θ for equilibrium. Given: P = 10 lb δ...
-
Multiple Choice Questions 1. In the long run, which of the following outcomes is most likely for a firm? a. Zero accounting profits but positive economic profits b. Zero accounting profits c....
-
In one Florida school district, students are rewarded for good grades and attendance with Happy Meals from McDonalds. Do you believe this type of reinforcement can help improve student grades and...
-
Web-based exercise. Tables of areas under a Normal curve, like Table B at the back of this book, are still common but are also giving way to applets that let you find areas visually. Go to the...
-
In 1966, Alfred Peet opened a shop selling coffee beans and loose teaand unknowingly started the gourmet coffee movement in America. Peets family had been in the coffee business in Holland; so Peet...
-
For the four unrelated situations, A-D, below, calculate the unknown amounts indicated by the letters appearing in each column: A B C D Beginning Assets $38,000 $12,000 $28,000 (d) Liabilities 18,600...
-
Using the following aqueous acid solution reduction potentials E (Pd 2+ ,Pd) = +0.915 V and E ([PdCl 4 ] 2 ,Pd) = +0.60 V, calculate the equilibrium constant for the reaction Pd+ (aq) + 4 Cl(aq)...
-
Use Fig. 6.12 to find the approximate potential of an aerated lake at pH = 6. With this information and Latimer diagrams from Resource section 3, predict the species at equilibrium for the elements...
-
What is the difference between profit or loss and other comprehensive income?LO1.
-
Construct a 90% confidence interval for the population standard deviation o at Bank B. Bank B 4.2 5.4 5.9 6.1 6.6 7.7 7.7 8.6 9.3 10.0
-
Jamila Traders has a head office in Nanyuki and an autonomous branch in Thika. The trial balances of the head office and the branch as at 30 September 2014 were as follows: Head office Sh. Sh. Thika...
-
Poll Results in the Media USA Today provided results from a survey of 1144 Americans who were asked if they approve of Brett Kavanaugh as the choice for Supreme Court justice. 51% of the respondents...
-
ROI analysis using the DuPont model a. Firm A has a margin of 7%, sales of $980,000, and ROI of 19.6%. Calculate the firm's average total assets. b. Firm B has net income of $259,200, turnover of...
-
The test statistic of z = - 2.93 is obtained when testing the claim that p < 2/ 3. This is a left-tailed test. Using a 0.01 significance level, complete parts (a) and (b). a. Find the critical...
-
Human Factors (December 2015) published a study on how well firefighter gloves fit. In a group of 586 firefighters who reported their glove size, the researchers determined whether the gloves fit...
-
Nate prepares slides for his microscope. In 1 day he prepared 12 different slides. Which equation best represents y, the total number of slides Nate prepares in x days if he continues at this rate? A...
-
For a [4Fe4S] protein, the following series of redox reactions are possible; each step is a 1-electron reduction or oxidation: (a) Which of these couples are accessible under physiological...
-
Describe how carbon fibres are manufactured. Summarize the properties and applications of carbon fibres and carboncarbon composites.
-
Describe the construction and operation of a lithiumion battery. Give examples of applications including those in the motor vehicle industry.
-
En prenant un exemple de votre choix, montrer comment on value un swap de taux de change.
-
How much would you need to invest today in order to receive: a. $10,000 in 5 years at 11%? b. $11,000 in 12 years at 8%? c. $12,000 each year for 10 years at 8%? d. $12,000 at the beginning of each...
-
A company that manufactures pulse Doppler insertion flow meters uses the Straight Line method for book depreciation purposes. Newly acquired equipment has a first cost of $190,000 with a 3-year life...

Study smarter with the SolutionInn App


