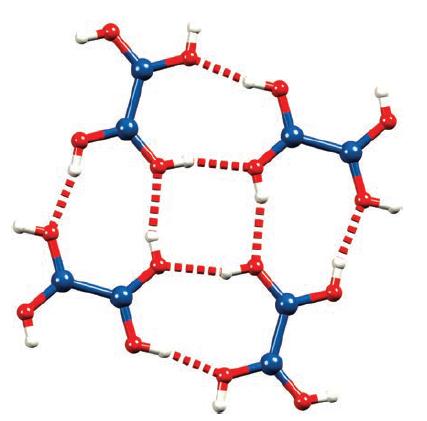
Figure 13.21 shows four hydrogen-bonded molecules of B 2 (OH) 4 . To what point group does
Question:
Figure 13.21 shows four hydrogen-bonded molecules of B2(OH)4. To what point group does a single molecule of B2(OH)4 belong?
Figure 13.21.
Transcribed Image Text:
.....
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
A single molecule of B2OH4 belongs to the point group D2h The point group of a molecule is the set o...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the molecule CH3Cl. (a) To what point group does the molecule belong? (b) How many normal modes of vibration does the molecule have? (c) What are the symmetries of the normal modes of...
-
(a) Draw a set of resonance structures for the hypothetical molecule PH 5 , ensuring that P obeys the octet rule in each structure. Assume a structure analogous to that of PF 5 . (b) To what point...
-
Determine what point group does PF 5 belong to, discuss how that point group is constructed, and discuss where do the linear and quadratic terms of that point group come from.
-
Kiev Corp. was incorporated on January 2, 2020, but was unable to begin manufacturing activities until July 1, 2020, because new factory facilities were not completed until that date. The Land and...
-
Trenton Fabrication Company purchased industrial tools costing $110,000, which fall in the 3-year property class under MACRS. Required: 1. Prepare a schedule of depreciation deductions assuming: a....
-
In Exercises sketch the plane curve and find its length over the given interval. Vector-Valued Function r(t) = 10 cos ti + 10 sin tj Interval [0, 2]
-
A contact that guarantees a certain percentage of profit is known as (a) incomplete contract (b) cost plus contracts (c) work-in-progress (d) finished goods.
-
1. Calculate the incremental, or marginal, cost per chair to LP of accepting the order from Southeast. 2. What assumptions did you make in calculating the incremental cost in Question 1? What...
-
Ruit Engineering Contractors incurred service salaries and wages of $47,600 (535.700 direct and $11.900 indirect) on an engineering project. The company applies overhead at arate of 22% of direct...
-
Figure 13.11c shows the solid state structure of the [Al(BH 4 ) 4 ] ion, present in [Ph 3 MeP][Al(BH 4 ) 4 ]. In the light of these structural data, account for the following observations, recorded...
-
Write equations for the following processes, involved in the extraction of the elements from their ores: (a) The reduction of boron oxide by Mg; (b) The result of the addition of hot aqueous NaOH to...
-
If the polar ice caps on Earth's solid surface were to melt, the oceans would be deeper. Strictly speaking, what effect would this have on Earth's rotation?
-
A baseball player's slugging percentage SLG can be calculated with the following formula (which is an example of a rational function): SLG = H+2B+2x(3B)+3x(HR) AB Q Image transcription text H+2B+2x...
-
Question During 2021, Cassandra Albright, who is single, worked part-time at a doctor's office and received a W-2. She also had a cash-basis consulting practice that had the following income and...
-
Shelly Beaman (social security number 412-34-5670) Is single and resides at 540 Front Street, Ashland, NC 27898. Shelly's W-2 wages Federal withholding Social security wages Social security...
-
P14-26. Forecasting with Parsimonious Method and Estimating Share Value Using the ROPI Model Following are income statements and balance sheets for Cisco Systems. CISCO SYSTEMS Consolidated...
-
A little lesson on horseracing.An exacta wager is where you pick the horse that you think will come first, and another who will come second. A trifecta wager is where you pick 3 horses that you think...
-
Knicks'N'Knacks produced 15,000 units in June at a cost of $39,250 In July, they produced 14,000 units at a cost of $37,500 In August, they produced 14,200 units at a cost of $36,500 Using the...
-
Choose two matrices A and B with dimension 2 x 2. Calculate det A, det B, and det (AB). Repeat this process until you are able to discover how these three determinants are related. Summarize your...
-
Draw the resonance structures for CO 3 2 .
-
Develop an argument based on bond enthalpies for the importance of SiO bonds, in preference to SiSi or SiH bonds, in substances common in the Earths crust. How and why does the behaviour of silicon...
-
(a) Use a molecular orbital program or input and output from software supplied by your instructor to construct a molecular orbital energy-level diagram to correlate the MO (from the output) and AO...
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


