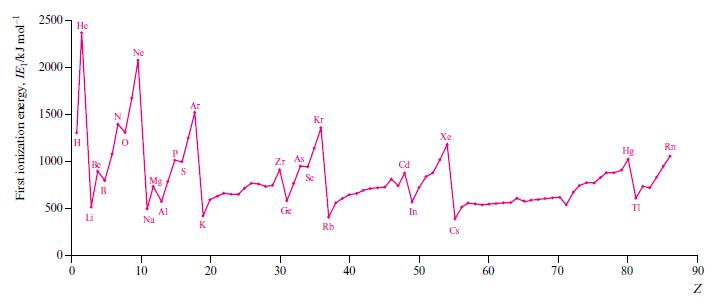
In Fig. 1.16, identify the trends in the first ionization energies of the elements in (a) Descending
Question:
In Fig. 1.16, identify the trends in the first ionization energies of the elements in
(a) Descending group 1,
(b) Descending group 13,
(c) Crossing the first row of the d-block,
(d) Crossing the row of elements from B to Ne,
(e) Going from Xe to Cs,
(f ) Going from P to S.
Rationalize each of the trends you have described.
Figure 1.16
Transcribed Image Text:
First ionization energy, IE,/kJ mol 2500 He 2000- 1500- 1000- 500- H Li B Mg Na 10 Al 20 Zr As 30 Kr Se Rb T 40 Cd In 50 Xe 60 70 Hg 11 80 Rn - N 90
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
In Figure 116 the trends in the first ionization energies of the elements can be observed The xaxis represents the atomic number Z and the yaxis repre...View the full answer

Answered By

User l_998468
I have extensive tutoring experience, having worked as a private tutor for over three years. I have tutored students from different academic levels, including high school, undergraduate, and graduate levels. My tutoring experience has taught me to be patient, attentive to student needs, and effective in communicating difficult concepts in simple terms.
I have a strong background in statistics, probability theory, data analysis, and data visualization. I am proficient in using statistical software such as R, Python, and SPSS, which are commonly used in academic research and data analysis. Additionally, I have excellent communication and interpersonal skills, which enable me to establish rapport with students, understand their learning styles, and adapt my teaching approach to meet their needs.
I am passionate about teaching and helping students achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Potassium and hydrogen react to form the ionic compound potassium hydride. (a) Write a balanced equation for this reaction. (b) Use data in Figures 7.10 and 7.12 to determine the energy change in...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-7. Ivan sold the following securities during the year and received a Form 1099-B that...
-
For the following exercises, use shells to find the volume generated by rotating the regions between the given curve and y = 0 around the x-axis. 130. y = 1-x,x = 0, and x = 1 131. y = x, x = 0, and...
-
IPW, Inc., began operations on January 1, 2012. The seven transactions recorded during January by the company accountant are shown in the following T-accounts: Complete the following table. For each...
-
a. When x 3 3x 2 + ax 7 is divided by x + 2, the remainder is 37. Find the value of a. b. When 9x 3 + bx 5 is divided by 3x + 2, the remainder is 13. Find the value of
-
3. Initial financing for internal service fund activities may be obtained from a Advances from another fund b Transfers from another fund c Transfer of related materials held by governmental...
-
Carmen Company is a manufacturer that completed numerous transactions during the month, some of which are shown below: a. Raw materials used in production as direct materials, $56,000. b. Paid direct...
-
please help me with these questions I'm stuck. Dividends Per Share Sandpiper Company has 15,000 shares of cumulative preferred 3% stock, $150 par and 50,000 shares of $20 par common stock. The...
-
Calculate the energy of the 3s atomic orbital of an H atom. Is the energy of the hydrogen 3p atomic orbital the same as or different from that of the 3s orbital?
-
Which of the following species are hydrogen-like: (a) H + ; (b) He + ; (c) He ; (d) Li + ; (e) Li 2+
-
a. Does a low credit rating necessarily imply that a bond is a bad investment? b. What factors besides the credit rating might be important in deciding whether a particular bond is a worthwhile...
-
How do these relevant legal principles apply: Duty of care Duty of obedience Duty of loyalty Shareholder Derivative suit Piercing the corporate veil...
-
what will you do as a hotel manager if a customer complained about bad service they received?
-
How do marketers use new products to maintain and grow their market share? Your response must include a specific example of a company that successfully grew its business or attracted a new target...
-
How do you encourage cross-functional synergy within your organization to break down silos and facilitate innovative solutions to complex challenges ?
-
1. what is intended internal resource strategies. How do you plan to develop or acquire resources (tangible and/or intangible) that would generate core competencies? What are examples of resource...
-
For the following data set (a) Construct a correlation matrix between x1, x2, x3, x4, and y. Is there any evidence that multicollinearity may be a problem? (b) Determine the multiple regression line...
-
How do the principles of (a) Physical controls and (b) Documentation controls apply to cash disbursements?
-
Identify the incorrect phrase in each of the following statements; explain your answer in each case. (a) Sodium dissolves in ammonia and amines to produce the sodium cation and solvated electrons or...
-
Which of the following pairs is most likely to form the desired compound? Describe the periodic trend and the physical basis for your answer in each case. (a) Ethanoate ion or edta 4 ion to react...
-
Summarize the chemistry of sodium that is being researched for the development of rechargeable sodium ion batteries. See M.D. Slater, D Kim, E. Lee, and C.S. Johnson, Adv. Func. Mater., 2013, 23, 947.
-
7 . 4 3 Buy - side vs . sell - side analysts' earnings forecasts. Refer to the Financial Analysts Journal ( July / August 2 0 0 8 ) study of earnings forecasts of buy - side and sell - side analysts,...
-
Bond P is a premium bond with a coupon of 8.6 percent , a YTM of 7.35 percent, and 15 years to maturity. Bond D is a discount bond with a coupon of 8.6 percent, a YTM of 10.35 percent, and also 15...
-
QUESTION 2 (25 MARKS) The draft financial statements of Sirius Bhd, Vega Bhd, Rigel Bhd and Capella for the year ended 31 December 2018 are as follows: Statement of Profit or Loss for the year ended...

Study smarter with the SolutionInn App


