Lanthanoid metal ions, Ln 3+ , can exchange with, and mimic the function of, Ca 2+ ions
Question:
Lanthanoid metal ions, Ln3+, can exchange with, and mimic the function of, Ca2+ ions in the human body. Lanthanum carbonate is administered as chewable tablets under the tradename of Fosrenol® to patients with particularly high levels of phosphate ions in the blood. However, there are significant gastrointestinal side-effects, and more soluble forms of lanthanide-containing drugs are being sought. Recent research in this field has investigated the reactions of Ln(NO3)3·6H2O with Hma (defined below) in the presence of NaOH or Et3N.
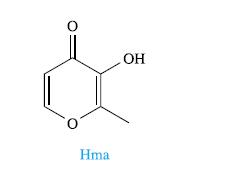
(a) Why are ligands with O-donor atoms chosen to bind Ln3+?
(b) What is the reason for adding base to the reaction mixture?
(c) The highest mass peaks in the electrospray ionization mass spectra of the complexes formed with La3+ and Eu3+ are at m/z 537 and 551, respectively. How do these peaks arise?
(d) Elemental analytical data for the Eu3+ complex are C 39.65, H 3.14%. What can you deduce from these data? Suggest a structure for the europium(III) complex.
(e) Complexes were formed with La3+, Eu3+, Gd3+, Tb3+ and Yb3+, but 1H NMR spectroscopic data were only reported for the La3+ complex. Suggest a reason for this.
(f) The Ln3+ complexes were subjected to hydroxyapatite-binding studies. Comment on the reasons for carrying out such investigations.
Step by Step Answer:






