From the following data at 298.15 K as well as data in Table 4.1 (Appendix B, Data
Question:
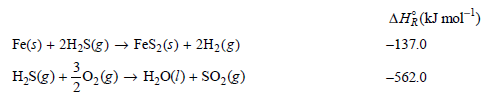
Transcribed Image Text:
AĦR(kJ mol) Fe(s) + 2H2S(g) –→ FeS2(s) + 2H2(g) -137.0 H;S(g) + 0,(g) → H,O() + SO,(E) -562.0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
HOl SOg HSg 20 8 Ss Og SO g Hg Og HO1 ...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the standard enthalpy of formation for diamond, given that C(graphite) + O2(g) CO2(g) Afr =-393.5 kJ/mol C(diamond) + O2(g) CO2(g) AF1 395.4 kJ/mol
-
From the following data at 298.15 K calculate the standard enthalpy of formation of FeO(s) and of Fe 2 O 3 (s): A(kJ mol) Fe,0;(s) + 3C(graphite) 2Fe(s) + 3cO(g) FeO(s) + C(graphite) Fe(s) + CO(g)...
-
From the enthalpy of formation for CO2 and the following information, calculate the standard enthalpy of formation for carbon monoxide (CO). Why can't we obtain it directly by measuring the enthalpy...
-
The following items are dropped from an airplane. Rank them in order from lowest terminal speed to highest and justify your ranking. (a) Bowling ball (b) Beach ball (c) Spear or javelin (pointing...
-
San Antonio Company produces accounting software. Its unit cost structure, based on an anticipated production volume of 150,000 units, is:sale price is 160, variable costs is 60 and fixed costs is...
-
Sketch the graph of the level surface (x, y, z) = c at the given value of c. (x, y, z) = x + 1/2y - z, c = 1
-
3 Give examples from your own experience of the cultural variability of gestures and facial expressions. Explain how this variability can lead to serious misunderstandings in cross-cultural...
-
In a production facility, 1.2-in-thick, 2-ft = 2-ft square brass plates (r = 532.5 lbm/ft3 and cp = 0.091 Btu/lbm ¢ °F) that are initially at a uniform temperature of 75°F are heated by...
-
Ratio Analysis Summary For Trucking Companies ( (Analyze this ratio chart and answer the following questions) 1.Which company is the most efficient in the use of their resources? 2.Which company is...
-
In a purely private market economy, how is the FOR WHOM question answered? Is that optimal?
-
Which of Ne or Ar has the larger van der Waals parameter a? Explain your reasoning.
-
Which of Ne or Ar has the larger van der Waals parameter b? Explain your reasoning.
-
Identify the tax issue or issues suggested by the following situations and state each issue in the form of a question. Two years ago, Ms. X loaned $3,500 to her 20-year-old daughter, who used the...
-
7. This is a question about electromagnetic waves. (a) Starting from Maxwell's equations in a vacuum show that the electric field E and magnetic field B obey wave equations and identify the velocity...
-
Participate in workplace health and safety Third- party report Task 1: Case scenario: Workplace hazard collection and risk control form You are required to review this workplace inspection form and...
-
The red curve is the position-time x-t graph for the ladybug. Each tick mark on the time axis of the graph marks off 0.5 s. Note: you can hit reset graph and graph again to watch the graph form again...
-
Oma's Bakery is thinking about replacing the convection oven with a new, more energy-efficient model. Information related to the old and new ovens follows: (Click the icon to view the information...
-
One could argue that substantial travel for work is an undesirable characteristic of any job. What would the theory of compensating differentials predict about the relative wages of a sales position...
-
Stellar, Inc., had depreciation expenses on its plant assets as follows for 2022, 2023, and 2024, respectively: $267,000, $289,000, and $368,000. Compute the trend percents for these years, assuming...
-
Differentiate the following terms/concepts: a. Personality types and money attitudes b. Planners and avoiders c. Moderating and adapting to biases d. "Perfectible judges" and "incorrigible judges"
-
A lot of information about the energy levels and wave functions of small inorganic molecules can be obtained from their ultraviolet spectra. An example of a spectrum with considerable vibrational...
-
Aromatic hydrocarbons and 12 form complexes from which charge transfer electronic transitions are observed the hydrocarbon acts as an electron donor and 12 as an electron acceptor the energies hvmax...
-
Consider some of the precautions that must be taken when conducting single-molecule spectroscopy experiments. (a) What is the molar concentration of a solution in which there is, on average, one...
-
crane Inc. common chairs currently sell for $30 each. The firms management believes that it's share should really sell for $54 each. If the firm just paid an annual dividend of two dollars per share...
-
Determine the simple interest earned on $10,000 after 10 years if the APR is 15%
-
give me an example of 10 transactions from daily routine that we buy and put for me Liabilities + Owners' Equity + Revenues - Expenses

Study smarter with the SolutionInn App


