From the following data at 298.15 K calculate the standard enthalpy of formation of FeO(s) and of
Question:
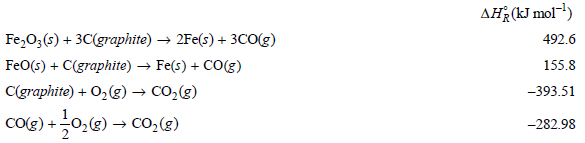
Transcribed Image Text:
AĦ¿(kJ mol) Fe,0;(s) + 3C(graphite) → 2Fe(s) + 3cO(g) FeO(s) + C(graphite) → Fe(s) + CO(g) C(graphite) + O2(g) → CO,(g) CO(e) +0,@) → Co,(3) 492.6 155.8 -393.51 -282.98
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (17 reviews)
Fes COg FeOs Cgraphite COg 1 0 8 Cgraphite Og COg ...View the full answer

Answered By

Gauri Hendre
I worked as EI educator for Eduphy India YT channel. I gave online tutorials to the students who were living in the villages and wanted to study much more and were preparing for NEET, TET. I gave tutions for topics in Biotechnology. I am currently working as a tutor on course hero for the biochemistry, microbiology, biology, cell biology, genetics subjects. I worked as a project intern in BAIF where did analysis on diseases mainly genetic disorders in the bovine. I worked as a trainee in serum institute of India and Vasantdada sugar institute. I am working as a writer on Quora partner program from 2019. I writing on the topics on social health issues including current COVID-19 pandemic, different concepts in science discipline. I learned foreign languages such as german and french upto A1 level. I attended different conferences in the science discipline and did trainings in cognitive skills and personality development skills from Lila Poonawalla foundation. I have been the member of Lila poonawalla foundation since 2017. Even I acquired the skills like Excel spreadsheet, MS Office, MS Powerpoint and Data entry.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the standard enthalpy of formation for diamond, given that C(graphite) + O2(g) CO2(g) Afr =-393.5 kJ/mol C(diamond) + O2(g) CO2(g) AF1 395.4 kJ/mol
-
From the following data at 298.15 K as well as data in Table 4.1 (Appendix B, Data Tables), calculate the standard enthalpy of formation of H 2 S(g) and of FeS 2 (s): AR(kJ mol) Fe(s) + 2H2S(g) ...
-
Calculate the standard enthalpy of formation of FeS 2 (s) at 600. °C from the following data at 298.15 K. Assume that the heat capacities are independent of temperature. You are also given that...
-
Suppose that today, you paid $1,000 for the bond described in Problem 8 of Chapter 8, The net present value functions: NPV and XNPV. What would be the bonds IRR? A bonds IRR is often called the yield...
-
Formulating an Argument about Earlier American Literature "What question at issue concerning earlier American literature has your research enabled you to identify and answer?" Looking ahead to the...
-
Solve each of the given inequalities algebraically. Graph each solution. |4 3x| 7
-
17. Suppose you are selecting a futures contract with which to hedge a portfolio. You have a choice of six contracts, each of which has the same variability, but with correlations of 0.95, 0.75,...
-
Rafferty was the principal shareholder in Continental Corporation, and, as a result, he received the lions share of Continentals dividends. Continental Corporation was eager to close an important...
-
Current Position Analysis Sherwood, Inc., the parent company of Tasty snack foods and Super beverages, had the following current assets and current liabilities at the end of two recent years: Current...
-
Item X is a standard item stocked in a companys inventory of component parts. Each year the firm, on a random basis, uses about 2,000 of item X, which costs $25 each. Storage costs, which include...
-
Compare the heat evolved at constant pressure per mole of oxygen in the combustion of sucrose (C 12 H 22 O 11 ) and palmitic acid (C 16 H 32 O 2 ) with the combustion of a typical protein, for which...
-
A camper stranded in snowy weather loses heat by wind convection. The camper is packing emergency rations consisting of 58% sucrose, 31% fat, and 11% protein by weight. Using the data provided in...
-
Dehumanization has been defined as a denial of humanness to others and has been associated with aggression and a host of negative consequences (Moller & Deci, 2009). In contemporary society, the use...
-
What is the logical ending point of a sequential game that starts at position (2,8) with player 1 moving first? Show your work. Player 1 Strategy B Strategy A Strategy A Player 2 Strategy B (3,4)...
-
Problem A-6 Income and Retained Earnings Statements Peanut Corporation is a private corporation using ASPE. At December 31, 2017, an analysis of the accounts and discussions with company officials...
-
8.5 Area Between Curves (dy) Calculus-Calculator Allowed Mastery Check #2 Name: Date: Period: For 1-2, find the area of the region bounded by the following curves. Show the integral set up with...
-
Your company has a travel policy that reimburses employees for the "ordinary and necessary" costs of business travel. Employees often mix a business trip with pleasure by either extending the time at...
-
Simulation A: 1 Diameter 600 mm 2 Focal Length 1800 mm 3 F/D Ratio 3 4 Eyepieces 30 m 5 Barlow? N 6 Celestial Sights M42 - M31 - M51 Simulation B: 1 Diameter 150 mm 2 Focal Length 1800 mm 3 F/D Ratio...
-
Graph y = g(x) by hand. g(x) = 1-zx x + 2x + 1
-
Which of the companies has the lowest accounts receivable turnover in the year 20X2? a. Company A. b. Company B. c. Company C. d. CompanyD. 20X1 20X2 Credit Sales Average Receivables Balance $1.0...
-
For how long on average would an atom remain on a surface at 400 K if its desertion activation energy were? (a) 20 kO mol-1, (b) 200 k] mol-I? Take TO = 0.12 ps. For how long on average would the...
-
A solid in contact with a gas at 8.86 kPa and 25C adsorbs 4.67 mg of the gas and obeys the Langmuir isotherm. The enthalpy change when 1.00 mmol of the adsorbed gas is desorbed is +12.2 J. What is...
-
Suppose it is known that ozone adsorbs on a particular surface in accord with a Langmuir isotherm. How could you use the pressure dependence of the fractional coverage to distinguish between...
-
Read the following and then answer the questions below:September 12: A Brisbane business offers by letter to sell 500 tyres to a New Zealand company. The Brisbane company does not specify a method of...
-
Fred returns home from work one day to discover his house surrounded by police. His wife is being held hostage and threatened by her captor. Fred pleads with the police to rescue her and offers...
-
Would like you to revisit one of these. Consideration must be clear and measurable.if you can't measure it then how can you show it has / has not been done?How can you sue someone for breach of...

Study smarter with the SolutionInn App


