Calculate the standard enthalpy of formation of FeS 2 (s) at 600. °C from the following data
Question:
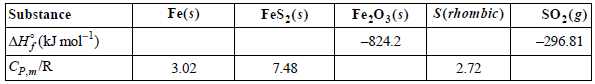
You are also given that for the reaction 2FeS2(s) + 11/2O2(g) †’ Fe2O2(s) + 4SO2(g).ˆ†HoR = - 1655 kJ mol-1
Transcribed Image Text:
Substance S(rhombic) so, (g) FeS:(s) Fe(s) Fe,03(s) -824.2 AH, (kJ mol-) -296.81 3.02 7.48 2.72 CP,/R
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (13 reviews)
2FeS 2 s 112O 2 gFe 2 O 2 s 4SO 2 gH o R 1655 kJ mol 1 1655 kJ mo...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the standard enthalpy of formation of NOCI (g) from the enthalpy of formation of NO given in Table 2.5, together with the following information: 2 NOCl (g) 2 NO (g) + Clz (g) 1 Ho = + Uo=...
-
The molar heat capacity of ethane is represented in the temperature range 298 K to 400 K by the empirical expression Cp,m/ (J K-1 mol-1) = 14.73 + 0.1272(T/K). The corresponding expressions for C(s)...
-
The head of a strike anywhere match contains tetraphosphorus trisulfide, P4S3. In an experiment, a student burned this compound in an excess of oxygen and found that it evolved 3651 kJ of heat per...
-
A factor used in measuring the loudness sensed by the human ear is (I/I 0 ), 0.3 where I is the intensity of the sound and I 0 is a reference intensity. Evaluate this factor for I = 3.2 10 6 W/m 2...
-
Write a short "employee Code of Conduct".
-
Solve the inequalities by displaying the solutions on a calculator. See Examples 9 and 10 in Section 17.2. Data from Example 10 of Section 17.2 Display the solution of the inequality 1 < 2x + 3 < 6...
-
5. Long-lived assets are purchased by a nongovernmental not-for-profit entity with cash that was restricted for that purpose. The assets are reported in temporarily restricted net assets....
-
A. The majority of the marketing managers have argued for a strategy that differentiates the hotel from local, high end hotels. B. These managers have focused on a strategy that positions the hotel...
-
Nakashima Gallery had the following petty cash transactions in February of the current year. Nakashima uses the perpetual system to account for merchandise inventory. February 2 Wrote a $350 check to...
-
Richard chooses technique 0 and 2 requiring 10+10-20 efforts and provising 10+11=21 benefits. Hence, 21 is returned as the output Example 2: input1: 3 input2: (10,10,10,10) input3: (10,11,12,15)...
-
Count the total number of s bonds and p bonds in the compound below: -3-N
-
Use the tabulated values of the enthalpy of combustion of benzene and the enthalpies of formation of CO 2 (g) and H 2 O(l) to determine H o f for benzene.
-
Using the given charge-to-mass ratios for electrons and protons, and knowing the magnitudes of their charges are equal, what is the ratio of the proton's mass to the electron's?
-
Kelly Corporation received an advanced payment of \(\$ 30,000\) in 2018 from Rufus Company for consulting services. Kelly performed half of the consulting in 2018 and the remainder in 2019. Kelly...
-
Rosa Dominguez, the owner of Elegant Dining in San Jose, California, is pondering whether to buy electronic menu technology and tablets for her five-star restaurant. Prices for a typical four course...
-
Dura Corporation makes metal frames for several world brands of portable home generators. They sell the frames to a wide variety of portable generator manufacturers such as DeWalt, DuroMax, Generac,...
-
Rocker Industries (RI) produces recreational in-line skates (see Exhibit 14.36). Demand is seasonal, peaking in the summer months, with a smaller peak demand during December. For one of their more...
-
The BOM, current inventory, and lead time (in months) for the in-line skates in Rocker Industries (A) case is shown in Exhibit 14.37. Using the chase demand strategy, you developed in Rocker...
-
Explain how to determine if two functions are inverses of each other.
-
The production budget of Artest Company calls for 80,000 units to be produced. If it takes 30 minutes to make one unit and the direct labor rate is $16 per hour, what is the total budgeted direct...
-
Calculate the magnitude of the diffusion-controlled rate constant at 298 K for a species in (a) Decylbenzene, (b) Concentrated sulfuric acid. The viscosities are 3.36 cP and 27 cP, respectively.
-
Calculate the magnitude of the diffusion-controlled rate constant at 298 K for the recombination of two atoms in benzene, for which 17 = 0.601 cP. Assuming the concentration of the reacting species...
-
For the gaseous reaction A + B --7 P, the reactive cross-section obtained from the experimental value of the pre-exponential factor is 8.7 X 10-22 nm. The collision cross-sections of A and B...
-
Saly paid $52,000 a year paid on a weekly basis. last pay she had $250 withheld in Income Tax, $48.97 for CPP and $15.80 for EI. an additional $and 25.00 in tax are deducted each pay. She allowed to...
-
Required information [The following information applies to the questions displayed below.] Dain's Diamond Bit Drilling purchased the following assets this year. Asset Drill bits (5-year) Drill bits...
-
Which of the following partnership items are not included in the self-employment income calculation? Ordinary income. Section 179 expense. Guaranteed payments. Gain on the sale of partnership...

Study smarter with the SolutionInn App


