Count the total number of s bonds and p bonds in the compound below: -3-N
Question:
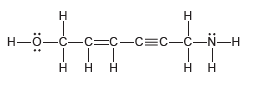
Transcribed Image Text:
н Н Н—ӧ—с—с-с—С3с—с-N—Н ннн н Н нн
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 69% (13 reviews)
Sixteen ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
(a) Show that the total number of atomic states (including different spin states) in a shell of principal quantum number n is 2n2. (b) Which shell has 50 states?
-
Figure gives a versus the sine of the angle in a single-slit diffraction experiment using light of wavelength 610 nm. The vertical axis scale is set by as = 12 rad. What are? (a) The slit width, (b)...
-
Figure gives the parameter B of Eq. 36-20 versus the sine of the angle in a two-slit interference experiment using light of wavelength 435nm. The vertical axis scale is set by s = 80.0 rad what are?...
-
Simplify the given expressions. Express all answers with positive exponents. 25/7/7/4 2/3 a
-
Write a report which should cover the Financial Statement Analysis for AT&T Inc. using Financial Ratios
-
Determine the values for x for which the radicals represent real numbers. 2 Vx - 3x
-
4. Contributed long-lived assets that are donor restricted for a certain time period are reported by a nongovernmental not-for-profit entity as: a Unrestricted support in unrestricted net assets b...
-
Gary Stewart and his wife Debbie, both age 32, have been married for twelve years and have a 2- year old son, Grant. Gary is a mid-level manager at a Fortune 500 company and currently earns $85,000...
-
Instructions On August 31, Baginski and Company receives its bank statement on below. The company deposits its receipts in the bank and makes all payments by check. The debit memo for $95 is for an...
-
Richard chooses technique 0 and 2 requiring 10+10-20 efforts and provising 10+11=21 benefits. Hence, 21 is returned as the output Example 2: input1: 3 input2: (10,10,10,10) input3: (10,11,12,15)...
-
Identify the expected hybridization state and geometry for the central atom in each of the following compounds: a. b. c. d. e. H. I-z: I-
-
Calculate the standard enthalpy of formation of FeS 2 (s) at 600. °C from the following data at 298.15 K. Assume that the heat capacities are independent of temperature. You are also given that...
-
What is the misappropriation theory?
-
The financial statements for the Columbia Sportswear Company can be found in Appendix A, and Under Armour, Inc.'s financial statements can be found in Appendix B at the end of this book. Required a....
-
Use the data from SE3-8 to prepare the closing entries for The Decade Company. Close the temporary accounts to income summary. The balance of \(\$ 8,500\) in the retained earnings account is from the...
-
Adjusting Entries The following selected accounts appear in the Birch Company's unadjusted trial balance as of December 31, the end of the fiscal year (all accounts have normal balances): Required...
-
Closing Entries Use the information provided in E3-5A to prepare journal entries to close the accounts using the Income Summary account. After these entries are posted, what is the balance in the...
-
Ceva, Inc. manufactures and services jet engines for air carriers. The engines cost \($10\) to \($40\) million each, depending on the specifications and plane. A 10-year service contract for a single...
-
Use the graph of g to solve Exercises 7176. Find g(-4). H y = g(x) 77 -5-4-3-2-1 # 32 31 TI y -3- DIIIIIIII x
-
What are conversion costs? What are prime costs?
-
Provide a mechanism that explains formation of the following products. CI HCI (concdCI+
-
Provide a mechanism that explains formation of the following products. OMe MeOH +CI OMet
-
Treating either 1-chloro-3-methyl-2-butene or 3-chloro-3-methyl-1-butene with Ag2O in water gives (in addition to AgCl) the following mixture of alcohol products. (a) Write a mechanism that accounts...
-
As a long-term investment at the beginning of the 2018 fiscal year, Florists International purchased 25% of Nursery Supplies Inc.'s 18 million shares for $66 million. The fair value and book value of...
-
Javier is currently paying $1,200 in interest on his credit cards annually. If, instead of paying interest, he saved this amount every year, how much would he accumulate in a tax-deferred account...
-
Your company is considering the purchase of a fleet of cars for $195,000. It can borrow at 6%. The cars will be used for four years. At the end of four years they will be worthless. You call a...

Study smarter with the SolutionInn App


