Ligand 20.12 forms an octahedral complex, [Fe(20.12) 3 ] 2+ . (a) Draw diagrams to show what
Question:
Ligand 20.12 forms an octahedral complex, [Fe(20.12)3]2+.
(a) Draw diagrams to show what isomers are possible.
(b) [Fe(20.12)3]Cl2 exhibits spin crossover at 120 K. Explain clearly what this statement means.
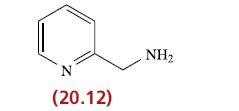
Transcribed Image Text:
N (20.12) NH₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
a When we consider an octahedral complex Fe2012 it is important to identify whether the ligand 2012 can form different isomers by binding in distinct ...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Case Study: Quick Fix Dental Practice Technology requirements Application must be built using Visual Studio 2019 or Visual Studio 2017, professional or enterprise. The community edition is not...
-
a. What do you understand by the term atomic orbital? b. Draw diagrams to show the shape of: i. An s orbital ii. A p orbital. c. Element x has the electronic configuration 1s 2 2s 2 2p 6 3s 2 3p 6 3d...
-
The equation of motion for a damped simple harmonic oscillator is ?where k and ? are constants, m is the mass and x is the displacement of the system. Describe the conditions for lightly damped,...
-
1. Give 3 examples of well-defined sets. 2. Name two subsets of the set of whole numbers using both the listing method and the rule method. 3. Let B [1, 3, 5, 7, 9}. List all the possible subsets of...
-
Project control should always focus on the critical path. Comment.
-
Making the sale to ?? is one of the keys to SCM success. Is the answer supplier or customer?
-
C8.3. Are currency translation gains and losses real gainsand losses to shareholders? Aren'ttheyjust an accounting effectthatis necessary to consolidate financial statementspreparedin different...
-
The trial balance and adjustments columns of the worksheet of Budget Business Systems at March 31, 2012, follow: Requirements 1. Compute the adjusted balance for each account that must be closed. 2....
-
Inventory Costing Methods The following data are for the Evans Company, which sells just one product:
-
(a) The values of max for the most intense absorptions in the electronic spectra of [CoCl 4 ] 2 and [Co(OH 2 ) 6 ] 2+ differ by a factor of about 100. Comment on this observation and state which...
-
Rationalize why the absorption spectrum of an aqueous solution of [T i (OH 2 ) 6 ] 2+ (stable under acidic conditions) exhibits two well-separated bands (430 and 650 nm) assigned to dd transitions,...
-
PFW Co. reports net income of $45,000. Partner salary allowances are Pitts $15,000, Filbert $5,000, and Witten $5,000. Indicate the division of net income to each partner, assuming the income ratio...
-
Show how you would go about balancing the following equations: Cu + HNO3 Cu(NO3)2 + NO + H2O HIO3 + Fel2 + HCI FeCl3 + ICI + H2O 2.Conservation of mass A student places 0.58 g of iron and 1.600 g...
-
Sales MOSS COMPANY Income Statement For Year Ended December 31, 2021 Cost of goods sold Gross profit Operating expenses (excluding depreciation) Depreciation expense Income before taxes Income taxes...
-
Prior to the Covid-19 epidemic, Master's and Ph.D. programs in psychology required applying students to submit their scores on the standardized graduate admission exam (GRE). For the past three...
-
Benicio wants to make sure that the Sales table does not contain any duplicate records, which would make any sales analysis incorrect. Identify and remove duplicate records in the Sales table as...
-
University Car Wash purchased new soap dispensing equipment that cost $261,000 including installation. The company estimates that the equipment will have a residual value of $27,000. University Car...
-
The following income statement and vertical analysis data are available for Westman Company: Westman Company Consolidated Income Statement s (In thousands) Required: 1. Suggest why net income...
-
Differentiate the following terms/concepts: a. Personality types and money attitudes b. Planners and avoiders c. Moderating and adapting to biases d. "Perfectible judges" and "incorrigible judges"
-
What reaction will take place if H 2 O is added to a mixture of NaNH 2 /NH 3 ?
-
Identify the configuration of the chirality centers shown below: a. b. c. d. -NH2 CH- H - - CH
-
Identify what reagents you would use to achieve each transformation: a. Conversion of 2-methyl-2-butene into a secondary alkyl halide b. Conversion of 2-methyl-2-butene into a tertiary alkyl halide...
-
ABC company makes turbo-encabulators, customized to satisfy each customers order. They split overhead into five pools, each with its own activity driver (direct labor for manufacturing, direct labor...
-
Variable manufacturing overhead becomes part of a unit's cost when variable costing is used.Group of answer choicesTrueFalse
-
Santa Fe Corporation has computed the following unit costs for the year just ended:Direct Material used $23Direct Labor $18Fixed selling and administrative cost $18Variable manufacturing overhead...

Study smarter with the SolutionInn App


