Potassium chromate is used as an indicator in titrations for the determination of chloride ion. At the
Question:
Potassium chromate is used as an indicator in titrations for the determination of chloride ion. At the end-point of a titration of an aqueous solution of a metal chloride salt (e.g. NaCl) against silver nitrate solution in the presence of potassium chromate, red Ag2CrO4 precipitates. Give equations for the pertinent reactions occurring during the titration, and, using relevant data from Table 7.5, explain how the indicator works.
Table 7.5
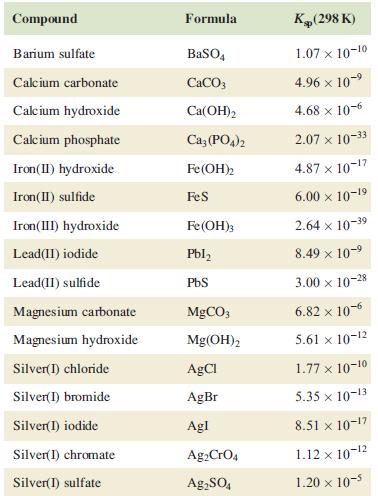
Transcribed Image Text:
Compound Barium sulfate Calcium carbonate Calcium hydroxide Calcium phosphate Iron(II) hydroxide Iron (II) sulfide Iron(III) hydroxide Lead(II) iodide Lead(II) sulfide Magnesium carbonate Magnesium hydroxide Silver(1) chloride Silver(1) bromide Silver(1) iodide Silver(1) chromate Silver(1) sulfate Formula BaSO4 CaCO3 Ca(OH)2 Ca3(PO4)2 Fe(OH)2 FeS Fe(OH)3 Pbl₂ PbS MgCO3 Mg(OH)2 AgCl AgBr Agl Ag2 CrO4 Ag₂SO4 K(298 K) 1.07 x 10-10 4.96 × 10-⁹ 4.68 x 10-6 2.07 x 10-33 4.87 x 10-¹7 6.00 × 10-19 2.64 x 10-3⁹ 8.49 × 10-⁹ 3.00 x 10-28 6.82 x 10-6 5.61 x 10-12 1.77 x 10-10 5.35 x 10-13 8.51 x 10-17 1.12 x 10-¹2 1.20 x 10-5
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
In the titration of a metal chloride salt eg NaCl with silver nitrate solution in the presence of potassium chromate the following reactions occur Formation of Silver Chloride Precipitate Agaq Claq Ag...View the full answer

Answered By

Churchil Mino
I have been a tutor for 2 years and have experience working with students of all ages and abilities. I am comfortable working with students one-on-one or in small groups, and am able to adapt my teaching style to meet the needs of each individual. I am patient and supportive, and my goal is to help my students succeed.
I have a strong background in math and science, and have tutored students in these subjects at all levels, from elementary school to college. I have also helped students prepare for standardized tests such as the SAT and ACT. In addition to academic tutoring, I have also worked as a swim coach and a camp counselor, and have experience working with children with special needs.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Ascorbic acid (vitamin C, page 354) reacts with I - 3 according to the equation Starch is used as an indicator in the reaction. The end point is marked by the appearance of a deep blue starch-iodine...
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
Rate Determination and Activation Energy An important part of the kinetic analysis of a chemical reactionis to determine the activation energy, E a .Activation energy can be defined as the energy...
-
What is the relationship between group norms and group cohesiveness? What roles do both cohesiveness and norms plan in shaping group performance?
-
Describe two benefits and two costs to a host country of FDI and to a home country of FDI.
-
SQL Injection and Cross-Site Scripting are leading Application Vulnerabilities which cause information leakage and unauthorized web application modification. Provide a list of 5 prevention techniques...
-
2. Voluntary health and welfare organizations: a Are required to use fund accounting principles to segregate unrestricted and restricted net assets b May report fund accounting information as...
-
Suppose Sparrow Sporting Goods Company reported the following data at March 31, 2012, with amounts in thousands: Use these data to prepare Sparrow Sporting Goods Company's income statement for the...
-
Sugar, Inc. sells $849,300 of goods during the year that have a cost of $588,600. Inventory was $31,683 at the beginning of the year and $35,938 at the end of the year. How long on average does it...
-
(a) Discuss the factors that contribute towards KCl being a readily soluble salt (35 g per 100 g H 2 O at 298 K). (b) Develop your answer to part (a) by using the following data: hyd H(K + , g) =...
-
(a) To what equilibria do the values of pK a (1) = 10:71 and pK a (2) = 7:56 for the conjugate acid of H 2 NCH 2 CH 2 NH 2 refer? (b) Calculate the corresponding values of pK b and write equations to...
-
Suppose that a pipe carrying a hot fluid with an external temperature of Ti and outer radius ri is to be insulated with an insulation material of thermal conductivity k and outer radius ro. Show that...
-
f. The coordinates of two points A and B are (1, 2) and (5,7) respectively. Find the equation and slope of the line AB. g. Find the rate of change of the area of a circle w.r.t its radius r when r =...
-
1. Sketch the anticipated pattern of cracks on the beam structure shown below. Assume that the structure is adequately reinforced for the load shown, and that the loads are large enough to cause...
-
Estimate the hydrogen consumption required to completely remove the sulfur from a hydrotreater feedstock and to reduce the nitrogen content of the product to 15 ppm by weight. The 48.5 API naphtha...
-
2. Consider the following kinds of information, and suggest the most appropriate data type to store or represent each: Information Suggested Data Type String A person's name A person's age in years A...
-
Steam at 32 MPa, 520C enters the first stage of a supercritical reheat cycle including three turbine stages. Steam exiting the first-stage turbine at pressure p is reheated at constant pressure to...
-
In a Gallup poll 513 national adults aged 18 years or older who consider themselves to be Republican were asked, "Of every tax dollar that goes to the federal government in Washington, D.C., how many...
-
Catherine (aged 42) and Johnson (aged 45) have been married for 12 years. Johnson is a project manager of an event company at a monthly salary of $55,000 with an additional one-month salary of...
-
What elementary row operations do the following matrices represent? What size matrices do they apply to? (a) (b) (c) (d) (e) 1 2 051 010 2 100 001 0301 00-0 1000
-
Identify the frontier orbitals of a Be atom in its ground state.
-
Account for the fact that the two Group 5 elements niobium (Period 5) and tantalum (Period 6) have the same atomic radii.
-
Provide a graph chart or data with sample numbers indicating Valuing Stocks and Bonds?
-
I just need help with part b. It says that the answer is not complete and some are wrong. So can you kindly fix it for me and give me the full answers as it says the answer is "not complete". Thank...
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...

Study smarter with the SolutionInn App


