(a) Discuss the factors that contribute towards KCl being a readily soluble salt (35 g per 100...
Question:
(a) Discuss the factors that contribute towards KCl being a readily soluble salt (35 g per 100 g H2O at 298 K).
(b) Develop your answer to part (a) by using the following data: ΔhydHº(K+, g) = −330 kJ mol−1;
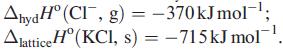
Transcribed Image Text:
AhydH° (CI, g) = -370kJmol-¹; Alattice H (KCl, s) = -715kJ mol
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 87% (8 reviews)
a Factors contributing towards KCl being a readily soluble salt Ionic nature KCl is an ionic compound consisting of K cations and Cl anions Ionic comp...View the full answer

Answered By

Collins Njuguna
I graduated from Maseno University with a Bachelor of Science in Applied Statistics. After graduation, I started tutoring students in mathematics. My experience in mathematics education is extensive and varied. I have taught a wide range of topics, including algebra, geometry, trigonometry, calculus, statistics, probability, and computer science. I have also worked with students of all ages and backgrounds, from elementary school to college.
My teaching method is based on the idea of hands-on learning. I believe that students learn best when they are actively engaged in the learning process, so I focus on giving students the tools they need to explore the material on their own. I also emphasize the importance of practice and review, as these are essential for mastering math concepts.
I have also developed several online and in-person courses on mathematics. My courses are designed to help students learn mathematics in an efficient and comprehensive way, and I use a variety of activities and exercises to ensure that my students are engaged and motivated.
Overall, my passion for mathematics and teaching has allowed me to be a successful tutor and educator in the field. I am confident that my experience will help your students master the mathematics they need to succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Suppose that the person with the above utility function is struck by lightning. He survives, except now his utility function is u = 1 log(x1 1) + 2 log(x2 2). Will his consumption decisions change...
-
The following table gives the systolic blood pressure (SBP), body size (QUET), age (AGE), and smoking history (SMK = 0 if a nonsmoker, SMK = 1 if a current or previous smoker) for a hypothetical...
-
A study was conducted on a sample of 53 patients presenting with prostate cancer who had also undergone a laparotomy to ascertain the extent of nodal involvement (Collett 1991). The result of the...
-
Access various employment Web sites (for example, www.monster.com and www.dice.com ) and find several job descriptions for a database administrator. Are the job descriptions similar? What are the...
-
In the United States, many states and cities deliberately seek investment in their states and communities by firms from other parts of the country. Why are some of those who seek investment from...
-
Find the term of containing 1/x 1/6 . 7 (x - ) V
-
E 22-3 Multiple choice 1. Which of the following statements is not required for nongovernmental voluntary health and welfare organizations that issue financial statements in accordance with GAAP? a...
-
Paisley Company prepared the following statement of cash flows for the current year: Required: Compute Paisley Companys free cash flow for the currentyear. Paisley Company Statement of Cash Flows-...
-
6744 2019 Escenario bsico 17: Cul es el monto de la deduccin estndar de Noah y Ella? $ ___
-
When NaCN dissolves in water, the resulting solution is basic. Account for this observation given that pK a for HCN is 9.31.
-
Potassium chromate is used as an indicator in titrations for the determination of chloride ion. At the end-point of a titration of an aqueous solution of a metal chloride salt (e.g. NaCl) against...
-
Access the Sampling Distribution for a Sample Mean web app and select Build Own as the shape for the population distribution. By typing numbers into the text field, you can create your own population...
-
Question 4 25 p J Mart is considering purchasing a new inventory control system featuring state-of-the-art technology. Two vendors have submitted proposals to supply J Mart with the new system. The...
-
ME 2352 Design Optimization Assignment TWO, due February 6th, 2024, 4:00 pm University of New Brunswick Department of Mechanical Engineering 1. By use of definition of linear dependency determine if:...
-
IKEA's People and Planet Positive sustainability plan, launched in 2012, aims to contribute to a better life for people and a better future for the planet. The plan outlines several sustainable goals...
-
Question 4 [25 marks] A cantilever beam AB is fixed to a wall and is subjected to concentrated and distributed loads as shown in figure B1. a) Draw the free-body diagram of the problem. [5 marks] a)...
-
GMC is an Australian farm machinery manufacturer, operating since 1975. The company makes high-quality farm machinery and equipment including a range of slashers, mowers, aerators, mulchers and...
-
Stocks may be categorized by sectors. Go to www.pearsonhighered.com/sullivanstats to obtain the data file 11_3_17 using the file format of your choice for the version of the text you are using. The...
-
Select the correct answer for each of the following questions. 1. On December 31, 20X3, Saxe Corporation was merged into Poe Corporation. In the business combination, Poe issued 200,000 shares of its...
-
Use the concept particularly the effects of penetration and shielding on the radial wavefunction, to account for the variation of single-bond covalent radii with position in the periodic table.
-
Discuss the following set of rate constants (k) and activation parameters for water exchange reactions of metal aqua ions. Species [TI(OH)]+ [V(OH)]+ [V(OH)]+ [Cr(OH)]+ [Cr(OH),(OH)]+ [Mn(OH)]+...
-
Given the following mechanism for the formation of a chelate complex, derive the rate law for the formation of the chelate. Discuss the step that is different from that for two monodentate ligands....
-
When preparing government-wide financial statements, the modified accrual based governments funds are adjusted. Please show the adjustments (in journal entry form with debits and credits) that would...
-
I need help finding the callable price and call value
-
On 31 October 2022, the owner took goods for his son as a birthday gift. The cost price of the goods was R15 000

Study smarter with the SolutionInn App


