Discuss the following set of rate constants (k) and activation parameters for water exchange reactions of metal
Question:
Discuss the following set of rate constants (k) and activation parameters for water exchange reactions of metal aqua ions.
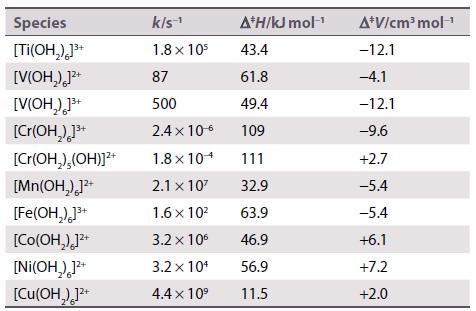
Transcribed Image Text:
Species [TI(OH₂)]³+ [V(OH₂)]²+ [V(OH₂)]³+ [Cr(OH₂)]³+ [Cr(OH₂),(OH)]²+ [Mn(OH₂)]²+ [Fe(OH₂)]³+ [Co(OH₂)]²+ [Ni(OH₂)]²+ [Cu(OH)]²+ k/s-¹ 1.8 x 105 87 500 2.4 x 10-6 1.8 x 104 2.1 x 10² 1.6 x 10² 3.2 x 10⁰ 3.2 x 10¹ 4.4 x 10⁹ A*H/kJ mol-¹ 43.4 61.8 49.4 109 111 32.9 63.9 46.9 56.9 11.5 A*V/cm³ mol-¹ -12.1 -4.1 -12.1 -9.6 +2.7 -5.4 -5.4 +6.1 +7.2 +2.0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
The set of rate constants k and activation parameters for water exchange reactions of metal aqua ions provides valuable insights into the kinetics and mechanisms of these reactions Lets discuss the si...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
As a specific biological example for Exercise 24.2 and Fig. E24.2(b), the synthesis of tryptophan can be described by the following set of material balances: where k 1 , k 2 , k 3 , and k 4 represent...
-
The following set of questions relates to using Poisson regression methods to analyze data from an in vitro study of human chromosome damage. In this study, using Poisson regression is appropriate...
-
The following set of data shows the number of alcoholic drinks that students at a Kansas university reported they had consumed in the past month: a. Assume the data set is a sample. Calculate the...
-
A U.S Company David Inc. sold merchandise to Fernando SA for 10,000 on September 14, 2017. The spot rate for euro is $0.720 on this day. On October 12, 2018, David Inc. purchased merchandise from...
-
At the end of Roshek Department Stores fiscal year on December 31, 2014, these accounts appeared in its adjusted trial balance. Freight-In ................$ 5,600 Inventory ................ 40,500...
-
The treasury stock in Question 14 is resold for $3,250,000. (a) What is the effect on the corporations revenue of the period? (b) What is the effect on stockholders equity?
-
Jamie Lee is attracted to the low monthly payment advertised for a vehicle lease. She may well be able to afford a more expensive car than she originally thought. Jamie Lee really needs to think this...
-
Diamond, an allotrope of carbon, is the hardest substance and the best conductor of heat yet characterized. For these reasons, diamond is used widely in industrial applications that require a strong...
-
10 Torino Company has 10,000 shares of $5 par value, 4% cumulative and nonparticipating preferred stock and 100,000 shares of $10 par value common stock outstanding. The company paid total cash...
-
Use the concept particularly the effects of penetration and shielding on the radial wavefunction, to account for the variation of single-bond covalent radii with position in the periodic table.
-
Given the following mechanism for the formation of a chelate complex, derive the rate law for the formation of the chelate. Discuss the step that is different from that for two monodentate ligands....
-
What is the surface area of a right rectangular cylinder with a radius of r and a height that is 1.5 times its diameter? 6r 2 + 4r 7r 2 8r 2 3r 3 + 2r 2 3r 3 + 4r
-
Identify a public conflict (such as a recent Congressional debate or even a celebrity breakup) that has come to the forefront in the media (or public's attention) in the last thirty days. You have...
-
Performance Management Issues You have been asked to return to your alma mater and speak to current students about performance management issues. To make the most of this experience for yourself and...
-
Analysis of competitor organization of our selected organization Walmart and its competitor Safeway. 1. Complete analysis of competitor organization; addresses all relevant factors and typically uses...
-
Defining Program Objectives of Youth centers Clearly define the objectives of your program or center. What specific outcomes do you hope to achieve? Examples may include promoting physical fitness,...
-
Identify a local or regional organization and analyze how they demonstrate servant leadership in their operations. You will want to review their website, social media, news, and other resources to...
-
Refer back to problem 23. Suppose Big Oil starts from the financing mix in Table 13.2, and then borrows an additional $200 million from the bank. It then pays out a special $200 million dividend,...
-
A 20-cm-square vertical plate is heated to a temperature of 30oC and submerged in glycerin at 10oC. Calculate the heat lost from both sides of the plate.
-
Predict the stereochemical outcome of radical bromination of the following alkanes: (a) (b) (c) (d)
-
Compound A has molecular formula C 5 H 11 Br. When compound A is treated with bromine in the presence of UV light, the major product is 2,2-dibromopentane. Treatmentof compound A with NaSH (a strong...
-
In calculating H o R at 285.15 K, only the H o f of the compounds that take part in the reactions listed in Tables 4.1 and 4.2 (Appendix B, Data Tables) are needed. Is this statement also true if you...
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


