Refer to Table 21.13 and calculate the rate constants for electron transfer in the oxidation of by
Question:
Refer to Table 21.13 and calculate the rate constants for electron transfer in the oxidation of
![]()
by the oxidants
(a)
![]()
(b)
![]()
Comment on the relative sizes of the rate constants.
Table 21.13.
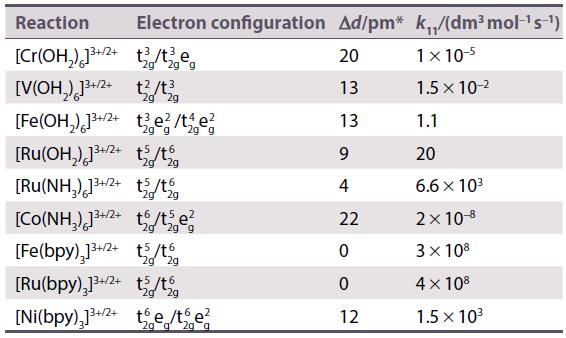
Transcribed Image Text:
[V(OH₂) 1²+ (E° (V³+/V²+) = -0.255V)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)
Based on the information provided in Table 2113 we can calculate the rate constants for electron tra...View the full answer

Answered By

Abdul Wahab Qaiser
Before working at Mariakani, I volunteered at a local community center, where I tutored students from diverse backgrounds. I helped them improve their academic performance and develop self-esteem and confidence. I used creative teaching methods, such as role-playing and group discussions, to make the learning experience more engaging and enjoyable.
In addition, I have conducted workshops and training sessions for educators and mental health professionals on various topics related to counseling and psychology. I have presented research papers at conferences and published articles in academic journals.
Overall, I am passionate about sharing my knowledge and helping others achieve their goals. I believe that tutoring is an excellent way to make a positive impact on people's lives, and I am committed to providing high-quality, personalized instruction to my students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
Hansel and Gretel are twins. Gretel goes to a star which is 65 light years away with a constant velocity plane (= v/c) = 0,88. Every birthday, the two do not forget to send each other radio signals,...
-
The photosynthetic reaction centre of the purple photosynthetic bacterium Rhodopseudomonas viridis contains a number of bound cofactors that participate in electron transfer reactions. The following...
-
The equilibrium Ac=' B + C at 25C is subjected to a temperature jump that slightly increases the concentrations of Band C. The measured relaxation time is 3.0 us. The equilibrium constant for the...
-
Define price and name the various types of prices described in this chapter.
-
Ryder Company's balance sheet shows Inventory $162,800. What additional disclosures should be made?
-
What is the contagion effect? Why is it more pronounced today than it was 30 years ago?
-
Analyzing Influences on Consumer Buying. Use advertisements, online articles, and personal observations to describe the economic, social, and personal factors influencing purchases of people in the...
-
Two items are omitted from each of the following three lists of cost of goods manufactured statement data. Determine the amounts of the missing items, identifying them byletter. 2,000 12,000 Work in...
-
The Board of Directors of pool company declared cash dividend of $1.50 per share on 42,000 shares of common stock on July 15, 2020 dividends to be paid on August 15, 2020 to stockholders of record on...
-
Solutions of [PtH 2 (PMe 3 ) 2 ] exist as a mixture of cis and trans isomers. Addition of excess PMe 3 led to formation of [PtH 2 (PMe 3 ) 3 ] at a concentration that could be detected using NMR....
-
Write the rate law for formation of [MnX(OH 2 ) 5 ] + from the aqua ion and X . How would you determine if the reaction is d or a?
-
The following five values are a sample: 11, 6, 10, 6, and 7. a. Compute the sample variance. b. Determine the sample standard deviation.
-
Pop Company holds 70% of Son Company stock. Pop has sold inventory to Son Company as follows: Percent of Sold Sales Inventory Cost to Price to Held at Year Pop Son Year end 2018 $203,000 $355,000 30%...
-
A B C D E F G H J K L 1 Cost Mortgage Payments 2 Cost Description The upscale hotel's building was acquired for $10 million, leading to monthly mortgage payments of $60,000. Behavior Dollar Amount...
-
What celebrity attributes make for effective celebrity product endorsements? Celebrity testimonials are advertising messages delivered by famous people who say or imply that they use the...
-
Initial investment of $100,000 in a new medical equipment. Interest Rate 10% (Borrowed money from a bank). Item Year 0 Year 1 Year 2 Year 3 Year 4 Year 5 Investment 100,000 Expected Cash Flow 20,000...
-
How do I draw a top view of this sketch and I was also wondering how to draw an oblique cabinet projection. + + 1 1 ' '
-
For each of the following pairs of issues stare which of the two you would expect to involve the lower proportionate underwriting and administrative costs, other things being equal: a. A large issue/...
-
Suppose that the electrical potential at the point (x, y, z) is E(x, y, z) = x + y - 2z. What is the direction of the acceleration at the point (1,3,2)?
-
Give an example of two systems separated by a wall that are in thermal but not chemical equilibrium.
-
A 1.50 mole sample of an ideal gas at 28.5C expands isothermally from an initial volume of 22.5 dm 3 to a final volume of 75.5 dm 3 . Calculate w for this process a. For expansion against a constant...
-
Derive the equation (H/T) V = C V + V/ from basic equations and definitions.
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...
Cuban Blindness Diary Of A Mysterious Epidemic Neuropathy 1st Edition - ISBN: 0128041196 - Free Book

Study smarter with the SolutionInn App


