Second order rate constants, k 2 , for the reaction of trans-[Pt(PEt 3 ) 2 Ph(MeOH)] +
Question:
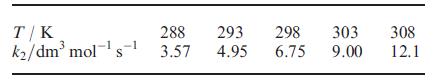
Second order rate constants, k2, for the reaction of trans-[Pt(PEt3)2Ph(MeOH)]+ with pyridine (py) in MeOH to give trans-[Pt(PEt3)2Ph(py)]+ vary with temperature as shown below. Use the data to determine the activation enthalpy and activation entropy for the reaction.
Transcribed Image Text:
T/K 288 293 298 k₂/dm³ mol-¹s¹ 3.57 4.95 6.75 303 9.00 308 12.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
To determine the activation enthalpy H and activation entropy S for a ...View the full answer

Answered By

Vishal madan
Experienced in Science is now likely available on social media here, only for you guys to explore my thoughts, thinking, opinions, and much more to guide humanity. My name is Hammad Shaukat. My greatest passion in life is teaching. I was born and raised in Pakistan (Rawalpindi) and experienced great success at school and in my higher education due to amazing and unforgettable teachers. This is the foundation of my commitment to helping my students, whatever their abilities may be. Currently, I am studying for a bachelor's degree specializing in physics. I have been tutoring and teaching for 2 years in various settings: tutoring small and large groups, private individual tutoring, and teaching in rural, suburban, and urban classroom and home settings. I am specializing in physics. I have much experience in science and am interested in astrology and cosmology. For instructors and tutors, you can mail at any time. ( SPECIALIZED IN SCIENCES ) Major subjects are sciences.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Determine the average Cp value in KJ/kg-K of a gas if 522 KJ/kg of heat is necessary to raise the temperature from 3OOK to 800K making the pressure constant * 1.038 1.026 1.440 1.044 What is the...
-
The equilibrium constant for the reaction of NH3 (aq) + H2O NH+4 + OH- is Kb = 1.479 10-5 at 5C 1.570 10 -5 at 10oC (a) Assuming H and S are constant in the interval 5 - 10C (probably a good...
-
The enthalpy of neutralization for the reaction of a strong acid with a strong base is 256 kJ/ mol of water produced. How much energy will be released when 200.0 mL of 0.400 M HNO3 is mixed with...
-
Explain Computer Network Types.
-
Xeriscape Nurseries, Inc., has four divisions. The corporations controller has been asked to prepare a cash budget for the Northern Division for the first quarter. Projected data supporting this...
-
Oil from an offshore rig located 3 miles from the shore is to be pumped to a location on the edge of the shore that is 8 miles east of the rig. The cost of constructing a pipe in the ocean from the...
-
What advice does the literature offer for creating, sustaining, and concentrating power in organizations? LO.1
-
Performance Plastics Company (PPC) has been operating for three years. The beginning account balances are: During the year, the company had the following summarized activities: a. Purchased equipment...
-
[The following information applies to the questions displayed below] A manufactured product has the following information for June Direct materials Direct labor Overhead Units manufactured Standard 5...
-
What structural features would you expect in the solid state of (a) Cs 2 [NpO 2 (acac) 3 ], (b) Np(BH 4 ) 4 , (c) The guanidinium salt of [ThF 3 (CO 3 ) 3 ] 5 , (d) Li 3 [LuMe 6 ]3DME, (e) Sm{CH(SiMe...
-
Prepare compound journal entries for each transaction. a. The owner, J. Cruz, invests $6,500 cash and $3,500 of equipment in the company. b. The company acquires $2,000 of supplies by paying $500...
-
Use the properties of infinite series to evaluate the following series. 2 3k 6k k=0 8.
-
Can anyone explain me how to calculate the ROI using the HISTORICAL COST NBV, the formula my instructor wants me to use is ADJ CF - HIST DEP /ASSETTOTAL - ACC DEP. And for the ROI of CURRENT COST NBV...
-
Consider the circuit to the right 3. If the total voltage supply in the circuit is 120V, and each resistor has a resistance of 400, what will the current read on each ammeter? |1= 12= 3 = 4. What...
-
1. The theory predicts the proportion of beans, in the four groups A, B, C and D should be 9:3:3:1. In an experiment among 1600 beans, the numbers in the four groups were 882, 313, 287 and 118. Does...
-
Would you recommend criminal charges in this case ( the screenshots below) and, if so, exactly which statutes against which person? Explain your reasoning (how the elements of the crime are met or...
-
check if each transaction is placed in the right place in each of the reports below and if there are any other mistakes in the different accounts after the first image which is a description of the...
-
What is Construction Work-in-Progress?
-
Assume a simple Keynesian depression economy with a multiplier of 4 and an initial equilibrium income of $3,000. Saving and investment equal $400, and assume full employment income is $4,000. a. What...
-
Using toluene as your only source of carbon atoms, show how you would prepare the following compound.
-
Starting with isopropyl benzene, propose a synthesis for acetophenone.
-
Propose a plausible synthesis for the following transformation.
-
Sociology
-
I am unsure how to answer question e as there are two variable changes. In each of the following, you are given two options with selected parameters. In each case, assume the risk-free rate is 6% and...
-
On January 1, Interworks paid a contractor to construct a new cell tower at a cost of $850,000. The tower had an estimated useful life of ten years and a salvage value of $100,000. Interworks...

Study smarter with the SolutionInn App


