Suggest products of the following reactions, which are not necessarily balanced on the left-hand side: (a) AlMe6+
Question:
Suggest products of the following reactions, which are not necessarily balanced on the left-hand side:
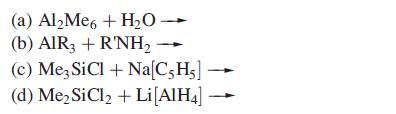
Transcribed Image Text:
(a) Al₂Me6+ H₂O (b) AIR3 + R'NH₂ (c) Me3 SiCl + Na[C5H5] (d) Me₂ SiCl₂ + Li [AIH4]
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
a Al2Me6 H2O The reaction involves the reaction of Al2Me6 dimethylaluminium with water H2O The gener...View the full answer

Answered By

User l_1006857
I am a computer science professional with expertise in databases, AI programming, data structures and algorithms, and mathematics. With a strong background in these areas, I possess the knowledge and skills necessary to design and optimize database systems, develop intelligent algorithms and models, and solve complex computational problems. My proficiency in SQL, NoSQL, machine learning techniques, and mathematical concepts equips me to contribute to innovative projects and drive technological advancements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Suggest products for the following reactions (which are not necessarily balanced on the left-hand sides): (a) CsF+ XeF4 (b) SiO + XeOF4 (c) XeF + SbF5 (d) XeF6 + [OH] (e) KrF + HO-
-
Suggest products for the following reactions, which are not necessarily balanced on the left-hand side: (a) KrF + Au (b) XeO3 + RbOH 298 K (c) [XeC1] [SbF1] (d) KrF + B (OTeF5)3 (e) C6F-XeF +...
-
Suggest products of the following reactions, which are not necessarily balanced on the left-hand side: (a) Mg + C5H6 - (b) MgCl + LiR LiAlH4 (c) RBeCl
-
Use the following table, Present Value of an Annuity of 1 Period 8% 9% 10% 1 0.926 0.917 0.909 2 1.783 1.759 1.736 3 2.577 2.531 2.487 A company has a minimum required rate of return of 9 %. It is...
-
Flowers Corporation reported net cash provided by operating activities of $412,000, net cash used by investing activities of $250,000, and net cash provided by financing activities of $70,000. In...
-
1. What does the standard error of the estimate mean in regression analysis? 2. Does the standard error of the estimate tell us anything about the predictive accuracy of the regression model? Explain...
-
Why is SSE not necessarily a good measure of cluster quality?
-
Sally and Tom are married, have three dependent children, and file a joint return in 2015. If they have adjusted gross income (AGI) of $90,000 and itemized deductions of $10,000, what is their...
-
A project has annual cash flows of $4,000 for the next 10 years and then $6,000 each year for the following 10 years. The IRR of this 20-year project is 11.88%. If the firm's WACC is 11%, what is the...
-
(a) BrO has been detected in the emission gases from volcanoes (N. Bobrowski et al. (2003) Nature, vol. 423). Construct an MO diagram for the formation of BrO from Br and O atoms. Comment on any...
-
(a) A black precipitate forms when H 2 S is added to an aqueous solution of a Cu(II) salt. The precipitate redissolves when Na 2 S is added to the solution. Suggest a reason for this observation. (b)...
-
Like the traditional marketplace, the digital marketspace offers marketers opportunities to create time, place, form, and possession utility. How do you think Internet-enabled technology rates in...
-
1. What are the threats being faced by Indian General Insurance Ltd. (IGIL)? 2. What are its traditional strengths? What 'business definitions' should it follow while capitalizing on its traditional...
-
You go to discuss the incident and the client's claims with your supervisor. As you retell the incident, it is clear that your supervisor is not comfortable. You ask your supervisor for advice on the...
-
Case Study Two: Rawlings Rawlings is an American sports equipment manufacturing company based in Town and Country, Missouri, and founded in 1887. Rawings specializes in baseball equipment and...
-
The discussion is for Administrating organizational change course. (we should write 300 words) Discussion question is: Refer to table 6.4 in your book. Think of a time when you were introduced to...
-
Content: Identify at least two resources for each of the four critical sections in the course project: Strategic Planning, Healthcare Reimbursement, Revenue Cycle Process, and Reimbursement...
-
Dodge City Products borrowed $100,000 cash by issuing a 36-month, $120,880 zero coupon note on January 1, 2021. The note matures on December 31, 2023. Required: 1. Prepare the entry to recognize the...
-
How much more interest will be earned if $5000 is invested for 6 years at 7% compounded continuously, instead of at 7% compounded quarterly?
-
Identify whether you would use dilute sulfuric acid or concentrated sulfuric acid to achieve each of the following transformations. In each case, explain your choice. a. b. [H,SO,] - . [H,SO,] + H20
-
Under anaerobic conditions, glucose is broken down in muscle tissue to form lactic acid according to the reaction: C 6 H 12 O 6 (s) 2CH 3 CHOHCOOH(s). Thermodynamic data at T = 298 K for glucose and...
-
Draw a mechanism for each of the following transformations: a. b. c. ,t Dilute H,SO,
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


