Suggest products for the following reactions (which are not necessarily balanced on the left-hand sides): (a) CsF+
Question:
Suggest products for the following reactions (which are not necessarily balanced on the left-hand sides):
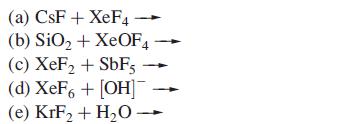
Transcribed Image Text:
(a) CsF+ XeF4 (b) SiO₂ + XeOF4 (c) XeF₂ + SbF5 (d) XeF6 + [OH] (e) KrF₂ + H₂O-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
a CSF XeF4 XeF3F CF4 b ...View the full answer

Answered By

Surendar Kumaradevan
I have worked with both teachers and students to offer specialized help with everything from grammar and vocabulary to challenging problem-solving in a range of academic disciplines. For each student's specific needs, I can offer explanations, examples, and practice tasks that will help them better understand complex ideas and develop their skills.
I employ a range of techniques and resources in my engaged, interesting tutoring sessions to keep students motivated and on task. I have the tools necessary to offer students the support and direction they require in order to achieve, whether they need assistance with their homework, test preparation, or simply want to hone their skills in a particular subject area.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Suggest products for the following reactions, which are not necessarily balanced on the left-hand side: (a) KrF + Au (b) XeO3 + RbOH 298 K (c) [XeC1] [SbF1] (d) KrF + B (OTeF5)3 (e) C6F-XeF +...
-
Suggest products of the following reactions, which are not necessarily balanced on the left-hand side: (a) AlMe6+ HO (b) AIR3 + R'NH (c) Me3 SiCl + Na[C5H5] (d) Me SiCl + Li [AIH4]
-
Suggest products of the following reactions, which are not necessarily balanced on the left-hand side: (a) Mg + C5H6 - (b) MgCl + LiR LiAlH4 (c) RBeCl
-
Lamonda Corp. uses a job order cost system. On April 1, the accounts had the following balances: The following transactions occurred during April: (a) Purchased materials on account at a cost of...
-
Using the information in P23-2B, compute the overhead controllable variance and the overhead volume variance.
-
Write a summary for 3 terminal.values where you explain why the specific 3 instrumental.values will.help you achieve them.
-
CJ4.1. Why is a simple forecast of operating income based on book value usually not a goodforecast? When might such a forecast be a goodforecast?
-
Odyssey Inc. has a total of $ 2,362,500 in production overhead costs. The companys products and related statistics follow. Additional data: The 330,000 pounds of material were purchased for $...
-
Need some help making T-account tables of debits and credits from each of these situations Problem 1: The client paid a $5,000 audit invoice which was expensed in the prior period out of their bank...
-
Equation 18.27 showed the preparation of [F 3 AsAuXe][Sb 2 F 11 ] from [F 3 AsAu][SbF 6 ]. Solid [F 3 AsAu][SbF6] contains a distorted [SbF 6 ] ion; one SbF bond is 193 pm, and five are in the range...
-
Comment on the following statements in terms of the properties of the elements mentioned. (a) For many decades, tungsten was used to make filaments in incandescent light bulbs. Tungsten is used in...
-
Counts of the type of automobile involved in police traffic stops Match this description of variables to a bar chart, Pareto chart, pie chart, or frequency table. Some are counts and others are...
-
1. Using appropriate examples, compare and contrast the genetic diversity of marine fish species with freshwater fish species (8 marks) 2. Your class went on a trip and discovered a crater lake on...
-
Find sin(29) given that cos(0) = and 0
-
Amazing Aquariums began as a class project on new business development. Now that the visionaries behind the idea have graduated, they want to explore their business idea and see if the concept could...
-
3. Modify the program of Example 05 so that, it takes and prints values using the following two functions respectively. void get (double *&a, int& n); void print (double *a, int n); 4. Following is a...
-
A company will be financing its operations with and a capital budget is P40,000,000 and a debt-to-equity ratio of 1. The interest rate on company's debt is 10%. The expected return on equity by the...
-
Service Company had net income during the current year of $65,800. The following information was obtained from Service's balance sheet: Accounts receivable ............................ $26,540...
-
Find a least expensive route, in monthly lease charges, between the pairs of computer centers in Exercise 11 using the lease charges given in Figure 2. a) Boston and Los Angeles b) New York and San...
-
In the mass spectrum of bromobenzene (Figure 15.27), the base peak appears at m/z = 77. Figure 15.27 a) Does this fragment contain Br? Explain your reasoning. b) Draw the cationic fragment that...
-
For each of the following compounds, determine whether the two protons shown in red are homotopic, enantiotopic, or diastereotopic: (a) (b) (c) (d) (e) OMe . CI H,
-
Butane (C 4 H 10 ) exhibits only two different kinds of protons, shown here in red and blue. (a) Explain why all four protons shown in red are chemically equivalent. (b) Explain why all six protons...
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


