The complex anion [FeCl 4 ] is yellow whereas the species [Fe 2 Cl 6 ]
Question:
The complex anion [FeCl4]− is yellow whereas the species [Fe2Cl6] trapped in an argon matrix is reddish. Dissolution of 0.1 mol FeCl3(s) in 1 dm3 of either POCl3 or PO(OR)3 produces a reddish solution that turns yellow on dilution. Titration of the red solution in POCl3 with Et4NCl solutions leads to a sharp colour change (from red to yellow) at a 1:1 mole ratio of FeCl3/Et4NCl. Vibrational spectra suggest that oxochloride solvents form adducts with typical Lewis acids by coordination of oxygen. Compare the following two sets of reactions as possible explanations of the observations.
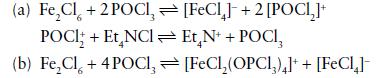
Both equilibria are shifted to products by dilution.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:





