The EI mass spectrum of lead(II) acetate shows four peak envelopes, each with an isotope pattern characteristic
Question:
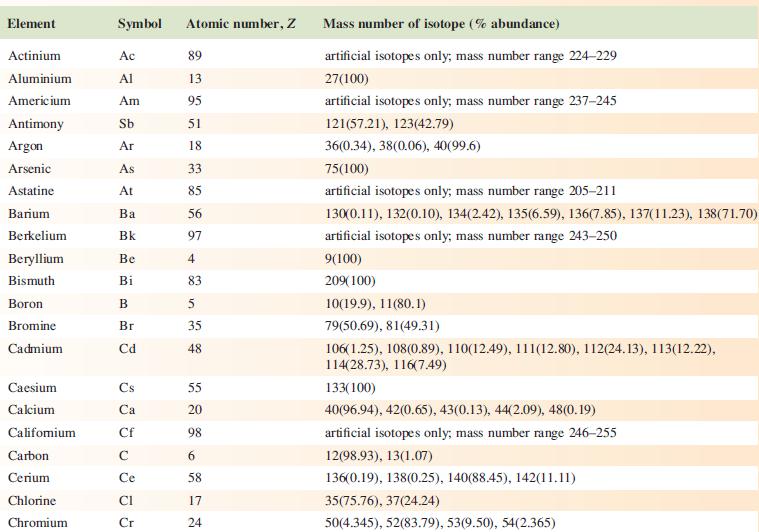
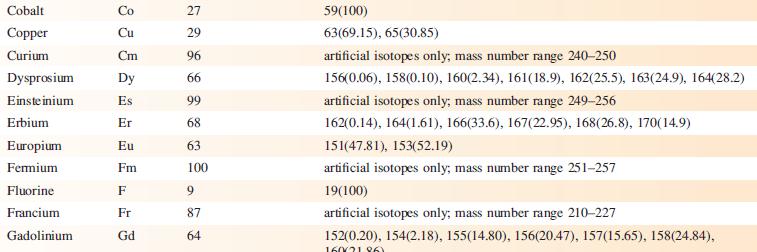
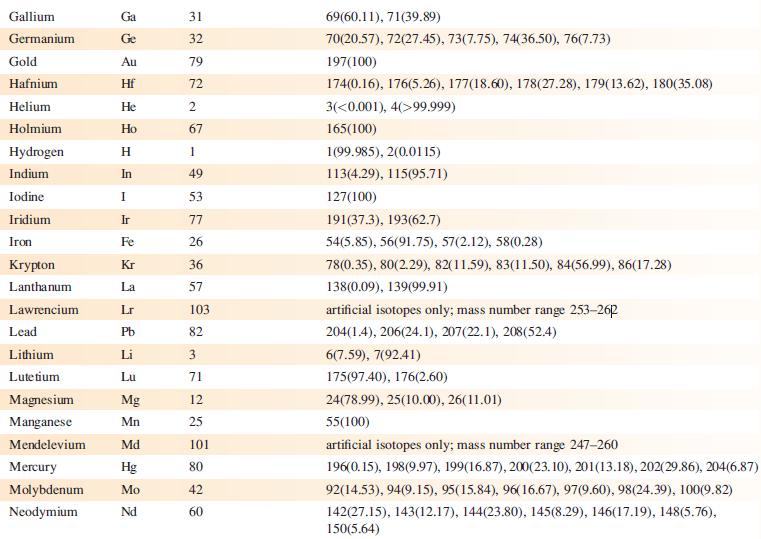
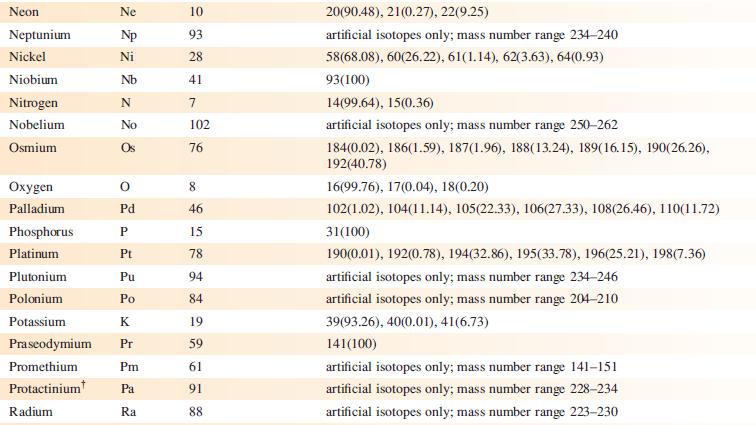
The EI mass spectrum of lead(II) acetate shows four peak envelopes, each with an isotope pattern characteristic of Pb. The most intense peak in each envelope appears at m/z 326.0, 267.0, 224.0 and 208.0, respectively.
(a) By using Appendix 5, sketch the pattern of each peak envelope.
(b) Assign the peaks.
Data from Appendix 5
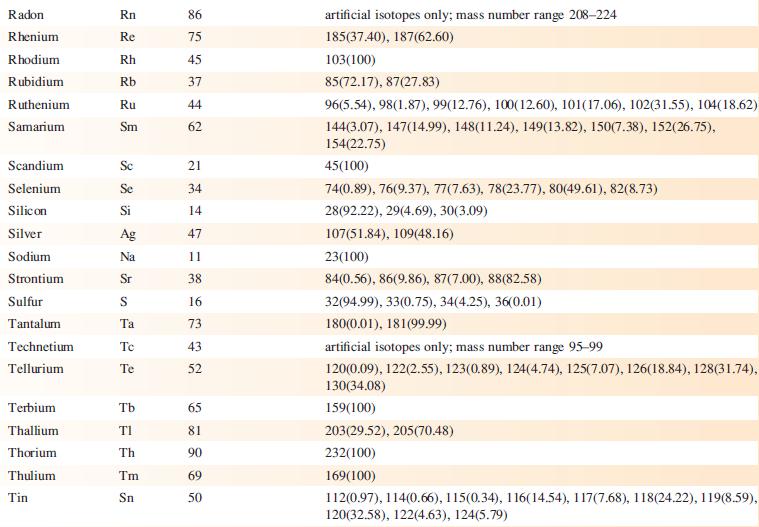
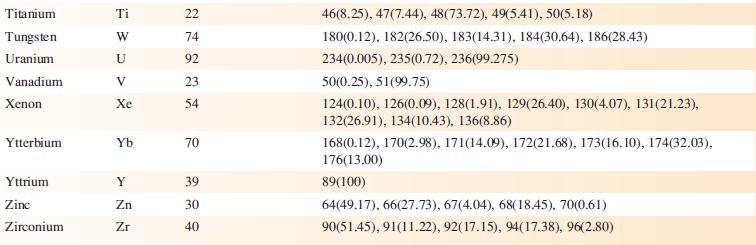
Transcribed Image Text:
Element Actinium Aluminium Americium Antimony Argon Arsenic Astatine Barium Berkelium Beryllium Bismuth Boron Bromine Cadmium Caesium Calcium Califomium Carbon Cerium Chlorine Chromium Symbol Atomic number, Z Ac Al Am Sb Ar As At Ba Bk Be Bi B Br Cd Cs Ca Cf с Ce CI Cr 89 13 95 51 18 33 85 56 97 4 83 5 35 48 55 20 98 6 58 17 24 Mass number of isotope (% abundance) artificial isotopes only; mass number range 224-229 27(100) artificial isotopes only; mass number range 237-245 121(57.21), 123(42.79) 36(0.34), 38(0.06), 40(99.6) 75(100) artificial isotopes only; mass number range 205-211 130(0.11), 132(0.10), 134(2.42), 135(6.59), 136(7.85), 137(11.23), 138(71.70)) artificial isotopes only; mass number range 243-250 9(100) 209(100) 10(19.9), 11(80.1) 79(50.69), 81(49.31) 106(1.25), 108(0.89), 110(12.49), 111(12.80), 112(24.13), 113(12.22), 114(28.73), 116(7.49) 133(100) 40(96.94), 42(0.65), 43(0.13), 44(2.09), 48(0.19) artificial isotopes only; mass number range 246-255 12(98.93), 13(1.07) 136(0.19), 138(0.25), 140(88.45), 142(11.11) 35(75.76), 37(24.24) 50(4.345), 52(83.79), 53(9.50), 54(2.365)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
Here are the visual representations of the isotope patter...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Rationalize the indicated fragments in the EI mass spectrum of each of the following molecules by proposing a structure of the fragment and a mechanism by which it is produced. (a)...
-
Suggest a structure for each of the ions corresponding to the following peaks in the EI mass spectrum of ethyl bromide, and give a mechanism for the formation of each ion. (The numbers in parentheses...
-
Propose reasonable fragmentation mechanisms that explain why The EI mass spectrum of benzoic acid shows major peaks at m/z = 105 and m/z = 77.
-
Two slot machines offer to double your money 3 times out of 5. Machine A takes $10 bets and Machine B takes $100 bets on each occasion. A risk-averse investor prefers to bet on A) Machine A B)...
-
Journalize the following transactions for the Pool Doctor. Explanations are not required. a. The Pool Doctor sold $63,000 of merchandise to By the River Spas, Inc., on account, terms 4/15, n/60. The...
-
In Exercises find the indefinite integral. 1 + cos sin a a - da
-
If in problem 16.5 the process is improved so the standard deviation is .0035, is the process capable now? LO.1
-
Use the information provided below to a. Compute the December 31, 2010 PBO and FMV of pension assets. b. Compute 2010 pension expense. c. Use the financial statements effects template to show the...
-
Tempo Company's fixed budget (based on sales of 12,000 units) for the first quarter reveals the following. Fixed Budget Sales (12,000 units $207 per unit) $ 2,484,000 Cost of goods sold Direct...
-
Both positive and negative-ion ESI mass spectra of [Me 4 Sb][Ph 2 SbCl 4 ] were recorded. In one spectrum, peaks at m/z 181 (100%), 182 (4.5%), 183 (74.6%) and 184 (3.4%) were observed. The other...
-
TheESImass spectrum(positivemode) of the compound shown below exhibits two peaks at m/z 299.2 (base peak) and 321.1. (a) What is a base peak? (b) Suggest how the observed peaks arise. [Data: C.J....
-
Infant-mortality rates are often used to assess quality of life and adequacy of health care. The rate is based on the number of deaths of infants under 1 year old in a given year per 1000 live births...
-
On October 1, Deloitte \& Coopers Price started a consulting firm. The asset, liability, and stockholders' equity account balances after each of the firm's first six transactions are shown below....
-
On June 1, a group of bush pilots in British Columbia, Canada, formed the Adventure Airlines, Inc., by selling \(\$ 51,000\) of common stock for cash. The group then leased several aircraft and...
-
During the first year of operation, 2011, Martin's Appliance recognized \$292,000 of service revenue on account. At the end of 2011 , the accounts receivable balance was \(\$ 57,400\). Even though...
-
During May, Willett Corp. purchased direct materials for 4,250 units at a total cost of \($61,625\). Willetts standard direct materials cost is \($14\) per unit. Prepare the journal entry to record...
-
Determine a positive real root of this equation using appropriate software: \[ 3.5 x^{3}-10 x^{0.5}-3 x=-4 \]
-
A sociologist believes that as the per capita personal incomes of states increase the birthrates decrease. She randomly selects eight states and the District of Columbia and obtains the following...
-
What are the principal alloying elements in SAE 4340 steel?
-
In their 2009 paper Icon of chemistry: The periodic system of chemical elements in the new century (Angew. Chem. Int. Ed., 2009, 48, 3404), S. Wang and W. Schwarz claim that the periodic system of...
-
According to the Born interpretation, the probability of finding an electron in a volume element dt is proportional to 2 d. (a) What is the most probable location of an electron in an H atom in its...
-
Survey the early and modern proposals for the construction of the periodic table. You should consider attempts to arrange the elements on helices and cones as well as the more practical...
-
On January 1, 2018, Brooks Corporation exchanged $1,259,000 fair-value consideration for all of the outstanding voting stock of Chandler, Inc. At the acquisition date, Chandler had a book value equal...
-
1. Determine the value of the right to use asset and lease liability at commencement of the lease.
-
Problem 22-1 The management of Sunland Instrument Company had concluded, with the concurrence of its independent auditors, that results of operations would be more fairly presented if Sunland changed...

Study smarter with the SolutionInn App


