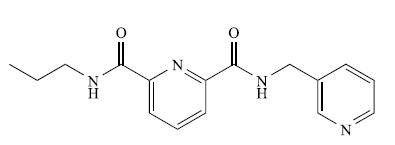
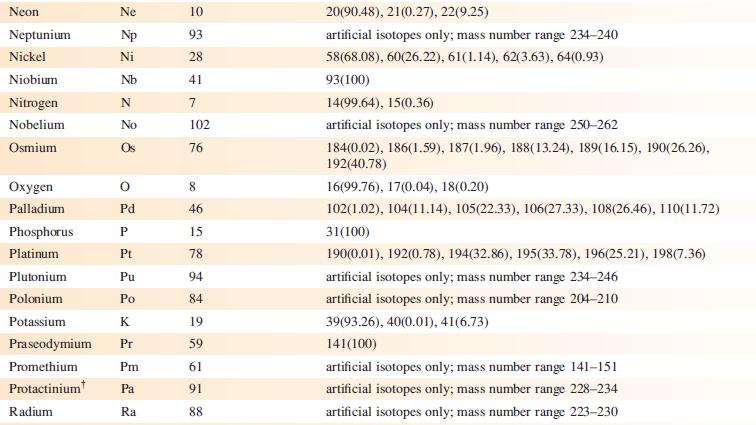
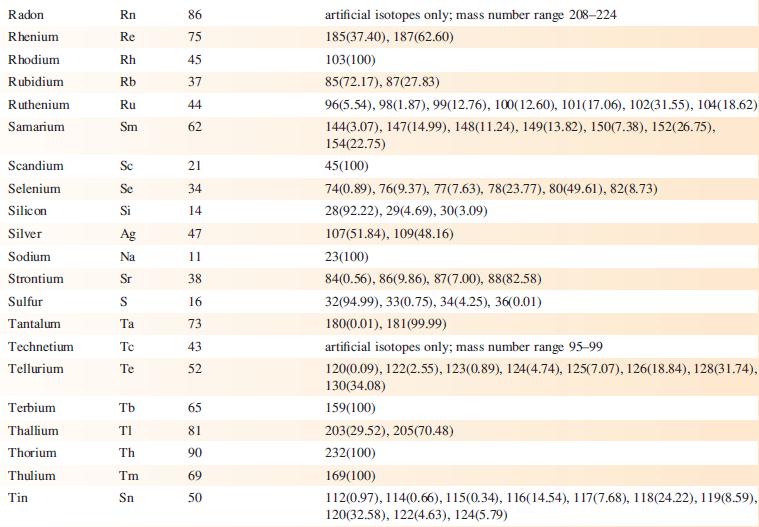
TheESImass spectrum(positivemode) of the compound shown below exhibits two peaks at m/z 299.2 (base peak) and 321.1.
Question:
TheESImass spectrum(positivemode) of the compound shown below exhibits two peaks at m/z 299.2 (base peak) and 321.1.
(a) What is a ‘base peak’?
(b) Suggest how the observed peaks arise. [Data: C.J. Sumby et al. (2009) Tetrahedron, vol. 65, p. 4681.]
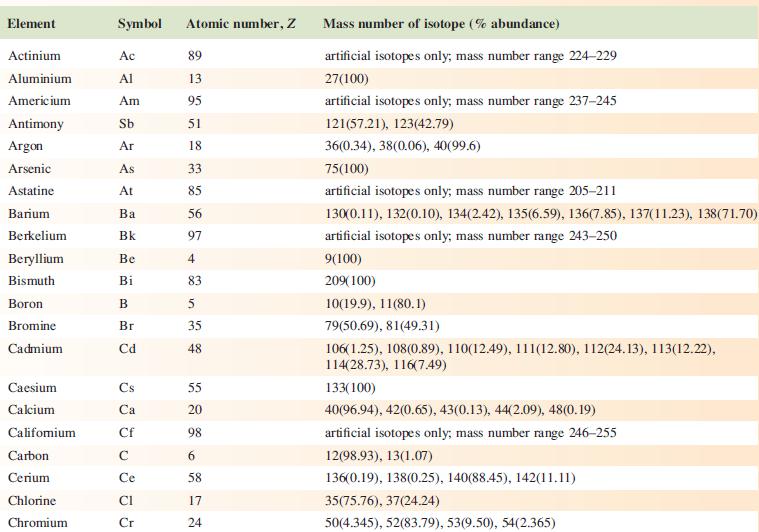
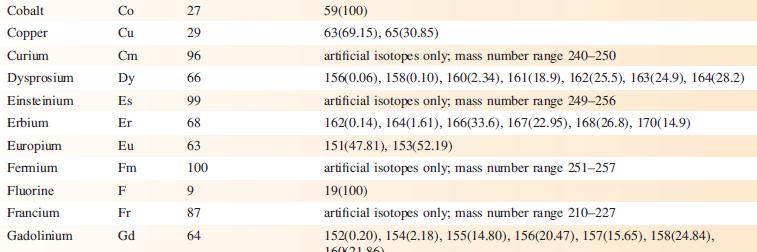
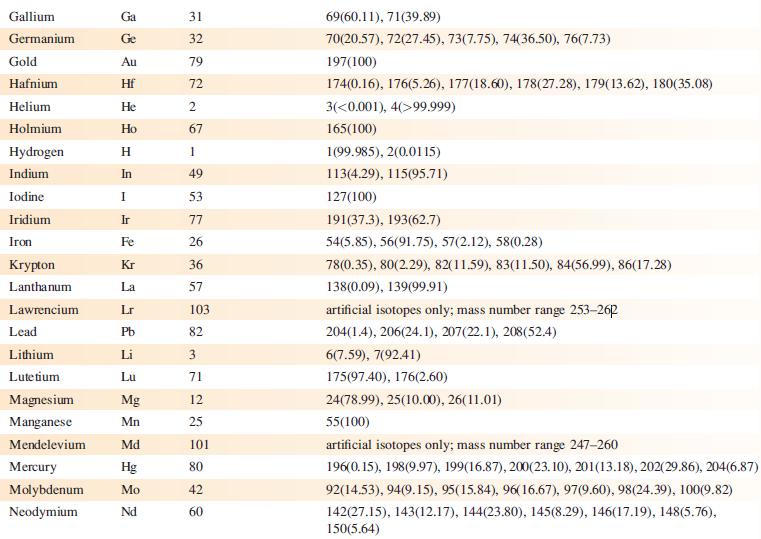
Data from Appendix 5
Transcribed Image Text:
H IZ IN Z O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
a What is a base peak In mass spectrometry the base peak is the peak with the highest intensity on the mass spectrum It is assigned a relative intensi...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In Problems 1542, solve each system of equations using Cramers Rule if it is applicable. If Cramers Rule is not applicable, write, Not applicable. x + 3 2 - + -3x + 3y - 2z 2 = -2 z = -5 5
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
The compound shown below has been advertised as a dietary supplement that purportedly prevents obesity, heart disease, and the ill effects of aging. Based on its structure, what physiological...
-
The Chief Financial Officer at Ford Motor Company is said to usea hybrid-costing system. Define the hybrid-costing system. Explainthe advantages to this company to use this system. I want a 10 page 2...
-
Journalize the following transactions for the Jamin Jimmys music store. a. Purchased $8,900 of merchandise on account, terms 3/15, n/30, FOB shipping point. b. Paid $180 to the freight company for...
-
In Exercises find the indefinite integral. (tan x)[In(cos x)] dx
-
In problem 16.6, what is the Cpk when: a. The process is centered on .75? Is the process capable? b. The process is centered on .74? Is the process capable? LO.1
-
Parque Corporation applied to Fairview Bank early in 2014 for a $400,000 five-year loan to finance plant modernization. The company proposes that the loan be unsecured and repaid from future...
-
Refer to the financial statements of The Home Depot in Appendix A . at the end of this book. ( Note: Fiscal 2 0 1 9 for The Home Depot runs from February 4 , 2 0 1 9 , to February 2 , 2 0 2 0 . As...
-
The EI mass spectrum of lead(II) acetate shows four peak envelopes, each with an isotope pattern characteristic of Pb. The most intense peak in each envelope appears at m/z 326.0, 267.0, 224.0 and...
-
The ESI mass spectrum (positive mode) of the complex shown below contained a peak envelope with m/z 527.9 (100%), 528.9 (15%), 529.9 (46%), 530.9 (7%), 531.9 (0.5%). A group of peaks of low intensity...
-
Explain the acronym CCCER.
-
You are the manager of internal audit of Coverit Corporation, a large insurance company. One day you receive an urgent letter from the controller expressing his concerns about some organizational...
-
Daintree Ltd. is a large retailer that operates department stores in all major cities throughout Australia. Recently it has expanded its operations into Southeast Asia. Although each store operates...
-
Draw two points P and Q. Then sketch PQ. Add a point R on the ray so that Q is between P and R. C D A B FL E
-
Hypothesis testing and testing claims with confidence intervals are two different approaches that lead to the same conclusion. In the following activities, you will compare and contrast those two...
-
The following system of periodic tasks is scheduled and executed according to a cyclic schedule. Draw an execution trace (timeline) showing two occurances of each task. Ti ei Pi 1 8 T2 4 15 T3 3 20...
-
Is a state with a higher population density likely to have a higher violent crime rate? The following data represent the population density (people per square mile) and violent crime rate (crimes per...
-
Ask students to outline the reasons why the various elements of culture (social structures and control systems, language and aesthetics, religion and other belief systems, educational systems, etc.)...
-
What is the ratio of the energy of an electronic ground-state He + ion to that of a Be 3+ ion?
-
In the paper What can the BohrSommerfeld model show students of chemistry in the 21st century? (M. Niaz and L. Cardellini, J. Chem. Educ., 2011, 88, 240) the authors use the development of models of...
-
(a) Construct the form of each molecular orbital in linear [HHeH] 2+ using 1s basis atomic orbitals on each atom and considering successive nodal surfaces. (b) Arrange the MOs in increasing energy....
-
Question 4. - Week 9. What are the major competitive issues General Electric faces when managing cooperative strategies? - (7 marks)
-
All of the following are roles of a derivative exchange EXCEPT: _____. A) maintaining margin requirements on futures contracts B) reducing the default risk on forward contracts C) performing daily...
-
you are analyzing the cost of debt for a firm. Do you know that the firms 14 year maturity, 7.8 Percent coupon bonds are selling at a price of $834. The Barnes pay interest semi annually. If these...

Study smarter with the SolutionInn App


