The industrial manufacture of NH 3 from N 2 and H 2 is carried out on a
Question:
The industrial manufacture of NH3 from N2 and H2 is carried out on a huge scale using heterogeneous catalysis, i.e. the reaction between gaseous N2 and H2 is carried out over a solid catalyst.
(a) Construct an MO diagram for N2 and use the diagram to explain why N2 is a chemically inert species.
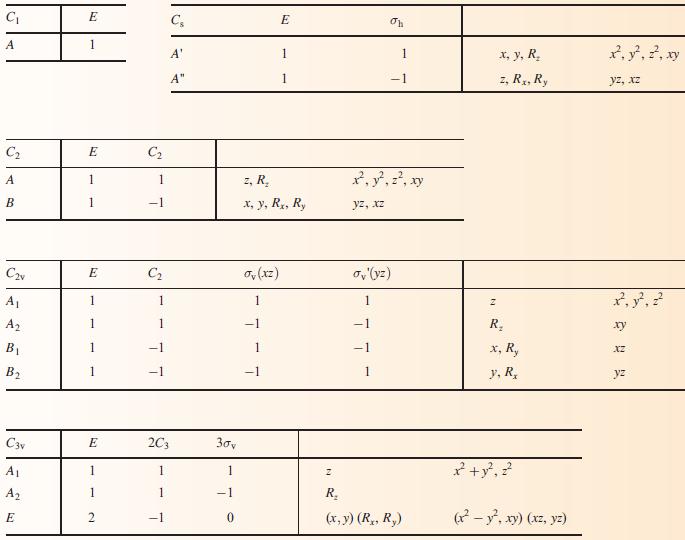
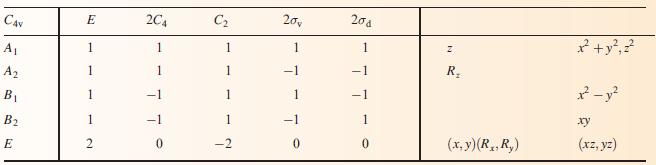
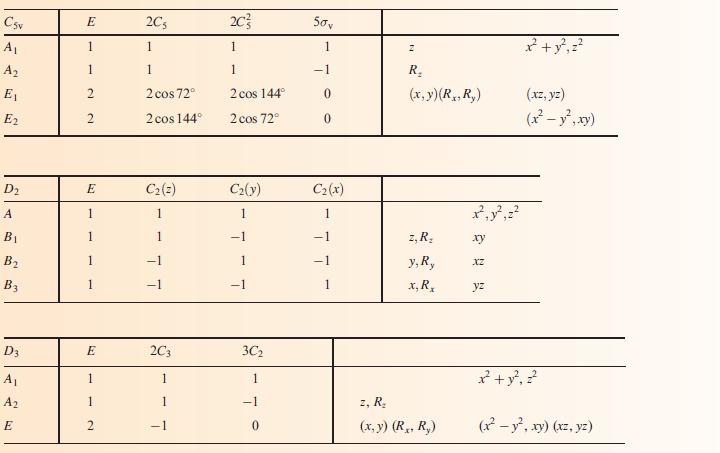
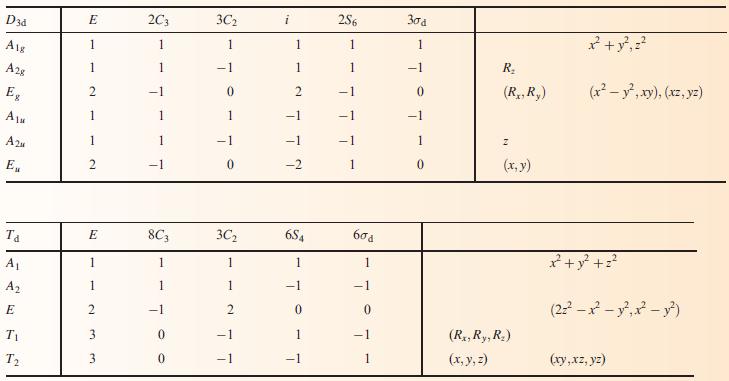
(b) To what point group does NH3 belong?
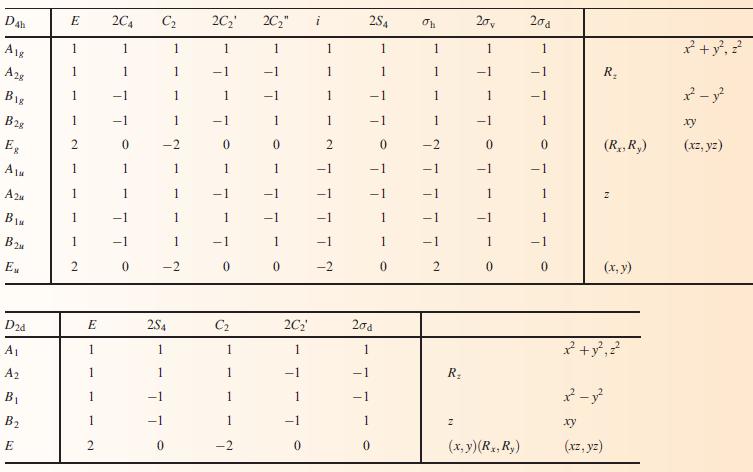
(c) Using the appropriate character table in Appendix 3, construct a set of ligand group orbitals for a triangular H3-fragment. Give symmetry labels to the LGOs.
(d) Construct an MO diagram for NH3 showing the interactions between the N atomic orbitals and the LGOs of the H3-fragment. Use the MO diagram to determine the N–H bond order and to confirm that NH3 is diamagnetic.
Data from Appendix 3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





