The iron content of a dietary supplement tablet was determined using atomic absorption spectrometry. A tablet (0.4878
Question:
The iron content of a dietary supplement tablet was determined using atomic absorption spectrometry. A tablet (0.4878 g) was ground to a fine powder and 0.1123 g was dissolved in dilute sulfuric acid and transferred to a 50 cm3 volumetric flask. A 10 cm3 sample of this solution was taken and made up to 100 cm3 in another volumetric flask. A series of standards was prepared that contained 1.00, 3.00, 5.00, 7.00, and 10.0 ppm iron. The absorptions of the standards and the sample solution were measured at the iron absorption wavelength.
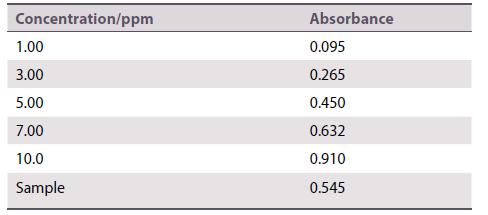
Calculate the mass of iron in the tablet.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:





