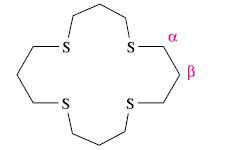
The ligand shown at the end of the problem, 16-S-4, forms the complex [Hg(16-S-4)] 2+ . The
Question:
The ligand shown at the end of the problem, 16-S-4, forms the complex [Hg(16-S-4)]2+. The solution 1H NMR spectrum of [Hg(16-S-4)][ClO4]2 consists of two signals at δ 3.40 and 2.46 ppm with relative integrals of 2:1. From the spectrum, the following coupling constants can be measured:
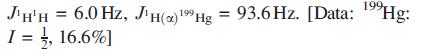
(a) Explain why complex formation between Hg(II) and S-donor ligands is particularly favoured.
(b) What coordination number do you expect for the Hg(II) centre in [Hg(16-S-4)]2+? On what basis have you made your choice?
(c) Sketch the 1H NMR spectrum of [Hg(16-S-4]-[ClO4]2.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





