The structure of cisplatin is shown below: Despite its success as an anticancer drug, the mechanism by
Question:
The structure of cisplatin is shown below:
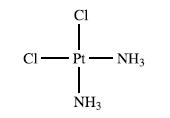
Despite its success as an anticancer drug, the mechanism by which the drug targets DNA in the body is not fully understood, although it is known that the nucleobase guanine (see Fig. 10.14) binds more readily to Pt(II) than the other nucleobases in DNA. Among model studies reported is that of the reactions of cisplatin and three related complexes with L-histidine and 1,2,4-triazole (Nu). The ligand substitutions occur in two, reversible steps:
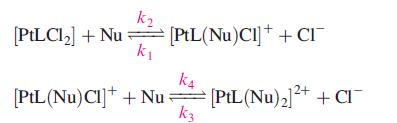
where L = (NH3)2, en, 1,2-diaminocyclohexane (dach), the deprotonated form, [MeCys]−, of S-methyl-L-cysteine (see Table 29.2 for L-cysteine). The reactions were investigated at 310K and pH 7.2 under pseudo-first order conditions.
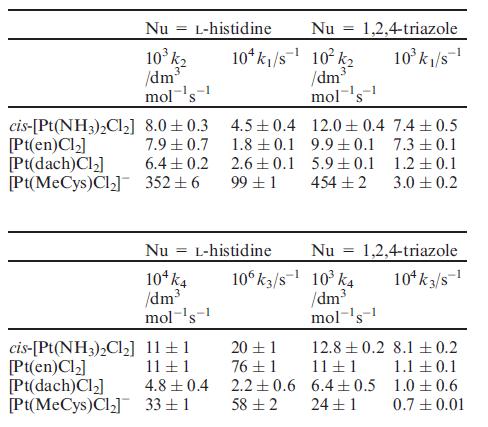
Rate constants for the reactions are as follows:
(a) Draw the structures of [Pt(en)Cl2], [Pt(dach)Cl2] and [Pt(MeCys)Cl2]−.
(b) Suggest why L-histidine and 1,2,4-triazole were chosen as nucleophiles in this study.
(c) Why were conditions of 310K and pH 7.2 used?
(d) Discuss the kinetic data, paying attention to and suggesting explanations for the trends in the relative rates of substitution.
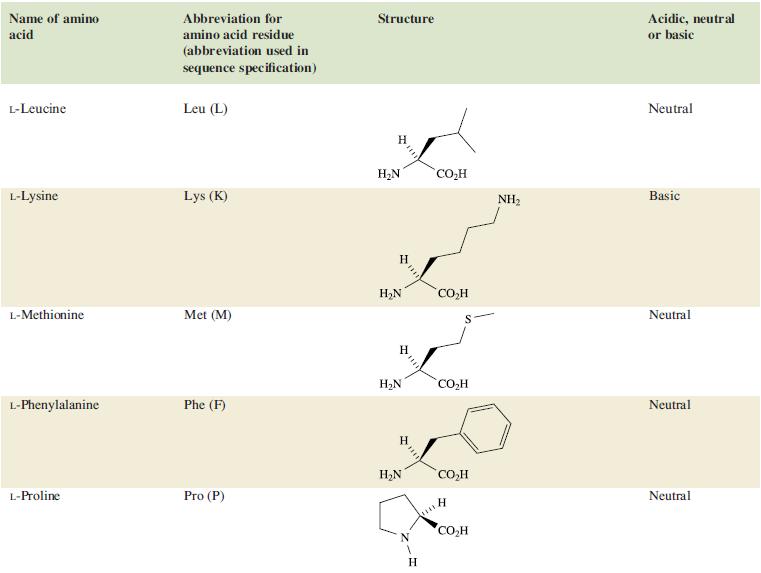
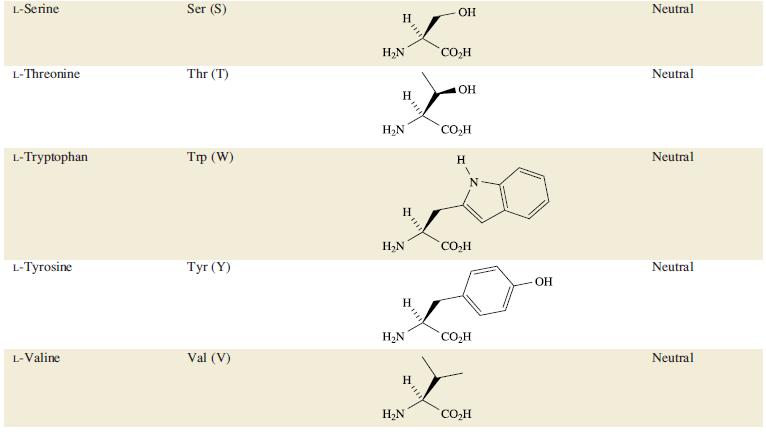
Figure 10.14.
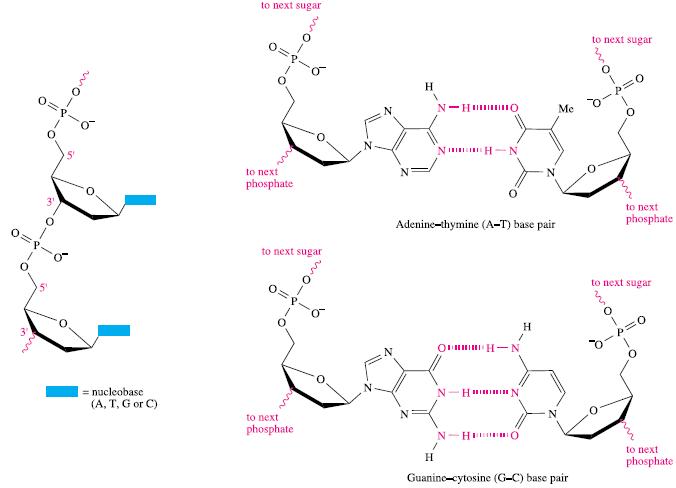
Table 29.2.
Step by Step Answer:






