The table below gives the average composition of the Earths atmosphere (ppm = parts per million). Water
Question:
The table below gives the average composition of the Earth’s atmosphere (ppm = parts per million). Water vapour is also present in small and variable amounts.
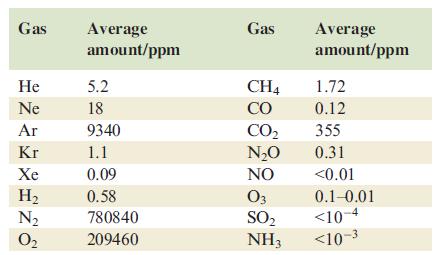
(a) Draw a Lewis structure for N2O, ensuring that each atom obeys the octet rule.
(b) Use the VSEPR model to predict the molecular shapes of SO2, NH3, N2O, CH4 and CO2.
(c) Which of the gases in the table are radicals? For each of the gases you have chosen, explain how the radical nature arises.
(d) O3 (ozone) is only present in
(e) Draw an MO diagram for the formation of N2 from two N atoms, using only the valence orbitals. Use the diagram to rationalize why N2 is chemically very inert.
(f) What is the relationship between the monoatomic gases in the Earth’s atmosphere?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:




