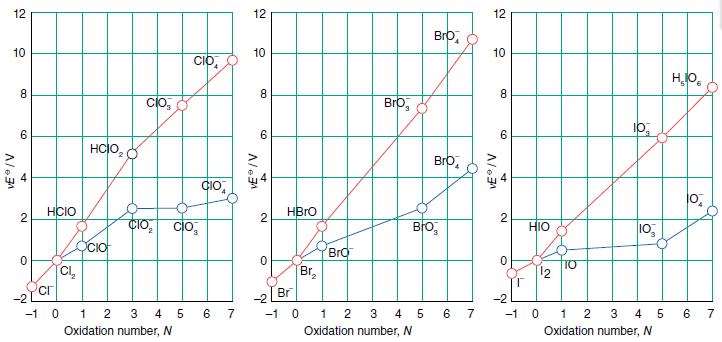
Use either the Frost diagram in Figure 17.14 or the Latimer diagrams in Resource section 3 to
Question:
Use either the Frost diagram in Figure 17.14 or the Latimer diagrams in Resource section 3 to calculate the standard potentials for the following couples in basic solution:
(a) ClO4−/ ClO− ,
(b) BrO4−/BrO− ,
(c) IO4− / IO−.
Comment on the relative feasibilities of the reduction reactions.
Figure 17.14.
Transcribed Image Text:
12 10 VEⓇ/V 8 6 2 0 HCIO СГ -1 0 2 Cl₂ HCIO, OCIO CIO CIO CIO₂ clo, CIO 1 2 3 4 5 6 7 Oxidation number, N 12 10 VEⓇ/V 8 6 4 2 0 HBrO Br -1 0 N õ Bro Bro Bro Bro BIO, 1 2 3 4 5 6 7 Oxidation number, N 12 10 VEⓇ/V 8 6 4 2 0 N -1 HIO 12 10 10₂ H₂O 10% 0 1 2 3 4 5 6 7 Oxidation number, N
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
To calculate the standard potentials for the given couples in basic solution pH 7 we can use the Latimer diagrams The standard potentials E for halfre...View the full answer

Answered By

User l_998468
I have extensive tutoring experience, having worked as a private tutor for over three years. I have tutored students from different academic levels, including high school, undergraduate, and graduate levels. My tutoring experience has taught me to be patient, attentive to student needs, and effective in communicating difficult concepts in simple terms.
I have a strong background in statistics, probability theory, data analysis, and data visualization. I am proficient in using statistical software such as R, Python, and SPSS, which are commonly used in academic research and data analysis. Additionally, I have excellent communication and interpersonal skills, which enable me to establish rapport with students, understand their learning styles, and adapt my teaching approach to meet their needs.
I am passionate about teaching and helping students achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Answer the following questions using the Frost diagram in Fig. 6.20. (a) What are the consequences of dissolving Cl 2 in aqueous basic solution? (b) What are the consequences of dissolving Cl 2 in...
-
The following Latimer diagrams show the standard reduction potentials E /V for some oxidation states of iron in acid and alkaline solution: (a) Plot a Frost diagram showing the states of Fe under...
-
Using the DJIA data in Problem 5-39, use exponential smooth with trend adjustment to forecast the opening DJIA value for 2014. Use α = 0.8 and β = 0.2. Compare the MSE for...
-
On a balance sheet, what valuation must be reported for short-term investments in trading securities?
-
The situation depicted in Fig. 24-5 is that of two tiny charged spheres separated by 10.0 cm in air. Find (a) The electric field E at point P, (b) The force on a 4.0 10 8 C charge placed at P, and...
-
2. Verify that ASae t satisfies the Black-Scholes PDE for a = 1 2 r 2 r 2 1 2 2 + 2(r ) 2
-
Cranston LTD. prepares its financial statements according to International Financial Reporting Standards. In October 2013, the company received a $2 million government grant. The grant represents 20%...
-
You are a senior auditor with Rodriguez & Jones, a small auditing firm located in Canterbury, an eastern suburb of Melbourne, Victoria. Your team has been assigned to the audit of a new client,...
-
Sketch all the isomers of the complexes [CrCl 4 F 2 ] 3 and [CrCl 3 F 3 ] 3 . Indicate how many fluorine environments would be indicated in the 19 F-NMR spectrum of each isomer.
-
Indicate the product of the reaction between ClF 5 and SbF 5 . Predict the shapes of the reactants and products.
-
The Covid-19 pandemic resulted in more accountants working from home as a standard business practice. Is this good or bad, and why?
-
The problem I have identified is that healthcare leaders could benefit from addressing the issue of stress and burnout, which impact revenue (Scott, 2022). I have found a peer-reviewed article...
-
Facebook, Inc is the company Complete a 3-5 year forecast for your target company assuming a 10% average growth rate for the duration of the forecast period Assuming a long-term growth rate of 5%...
-
BSC-It is important for healthcare leaders to link their departmental balanced scorecard (BSC) to a corporate BSC because it facilitates alignment with the overall strategic objectives of the...
-
Hebert Company adds material at the beginning of production. The following production information is available for March: Beginning Work in Process Inventory (40% complete as to conversion) Started...
-
What modifications would you suggest the leaders of the steel organization when dealing with the use of more efficient technology, carbon emissions, and negative economic impacts in order tomake in...
-
Read the scenario Katrina's Candies and suggest one (1) method in which Herb could use a cost-benefit analysis to argue for or against an expansion. Create three (3) optimal decision rules for...
-
Design a circuit which negative the content of any register and store it in the same register.
-
Lead-acid batteries accounted for 69% of all lead consumed in the US in 2015. (a) Complete the cell reaction given below (not balanced on the left-hand side) and show that the oxidation state changes...
-
Using bond enthalpy terms from Tables 14.2 and 15.3, estimate values of r H for the following reactions (a) 2N 2 N 4 (tetrahedral structure); (b) 2P 2 P 4 (tetrahedral structure); (c) 2C 2 H 2 C...
-
The glass industry manufactures millions of tonnes of glass per year. (a) Only certain element oxides form glasses. Explain why this is, giving examples of what are termed in the glass industry as...
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


