Use the data in Appendix 11 to rationalize quantitatively why: (a) Mg liberates H 2 from dilute
Question:
Use the data in Appendix 11 to rationalize quantitatively why:
(a) Mg liberates H2 from dilute HCl, but Cu does not;
(b) Br2 liberates I2 from aqueous KI solution, but does not liberate Cl2 from aqueous KCl solution;
(c) the role of Fe3+ ions as an oxidizing agent is influenced by the presence of certain ligands in solution;
(d) a method of growing Ag crystals is to immerse a zinc foil in an aqueous solution of AgNO3.
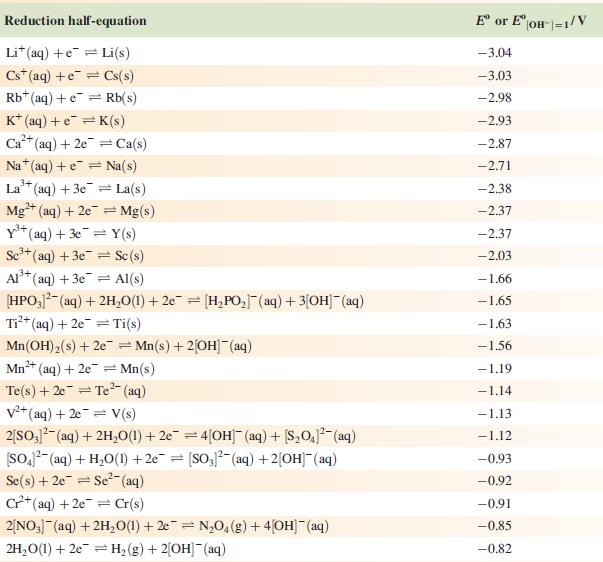
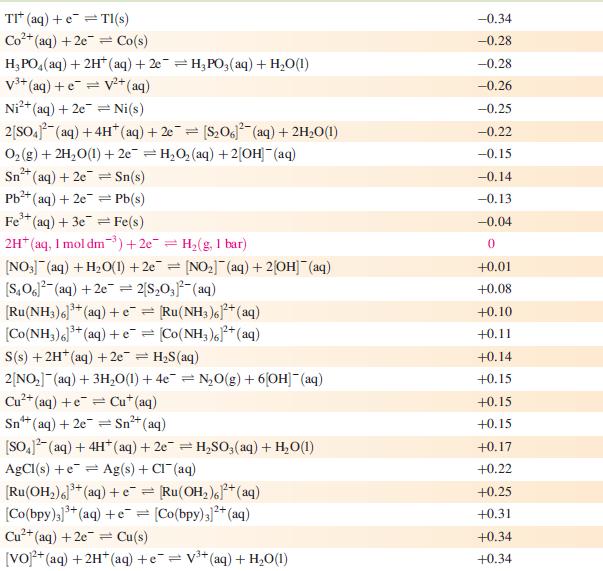
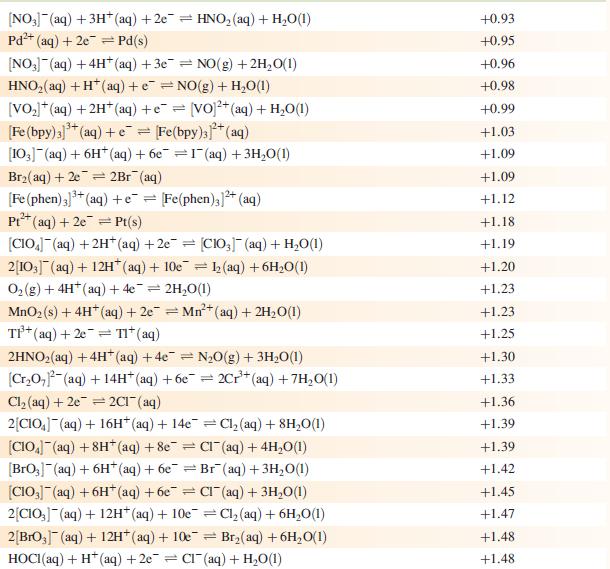
Data from Appendix 11
The concentration of each aqueous solution is 1 mol dm−3 and the pressure of a gaseous component is 1 bar (105 Pa). (Changing the standard pressure to 1 atm (101 300 Pa) makes no difference to the values of Eo at this level of accuracy.) Each half-cell listed contains the specified solution species at a concentration of 1 mol dm−3; where the half-cell contains [OH]−, the value of Eo refers to [OH−] = 1 mol dm−3, hence the notation Eo[OH−] = 1

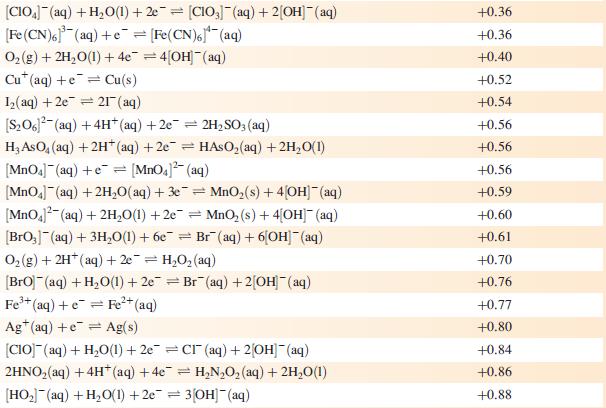

Step by Step Answer:






